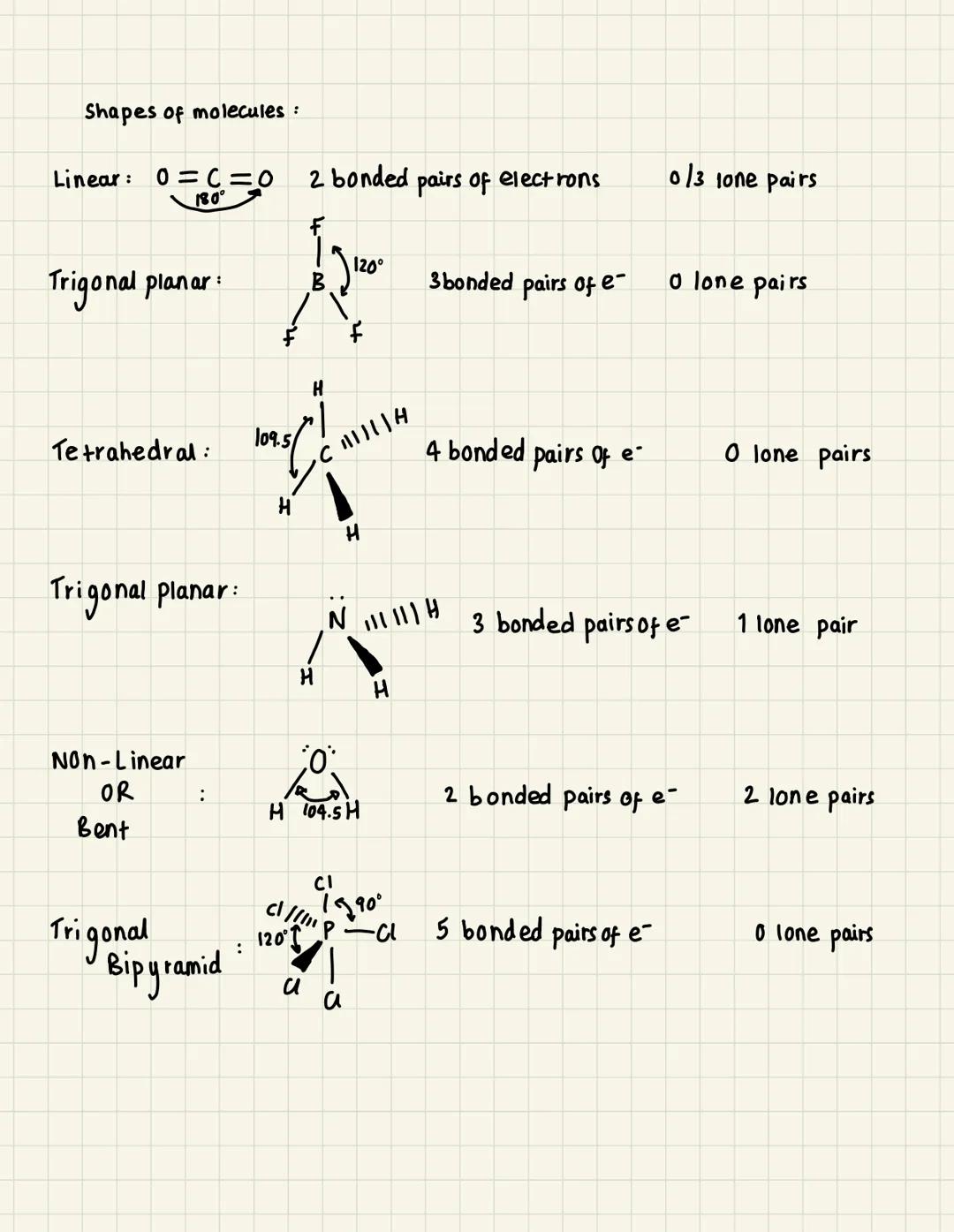
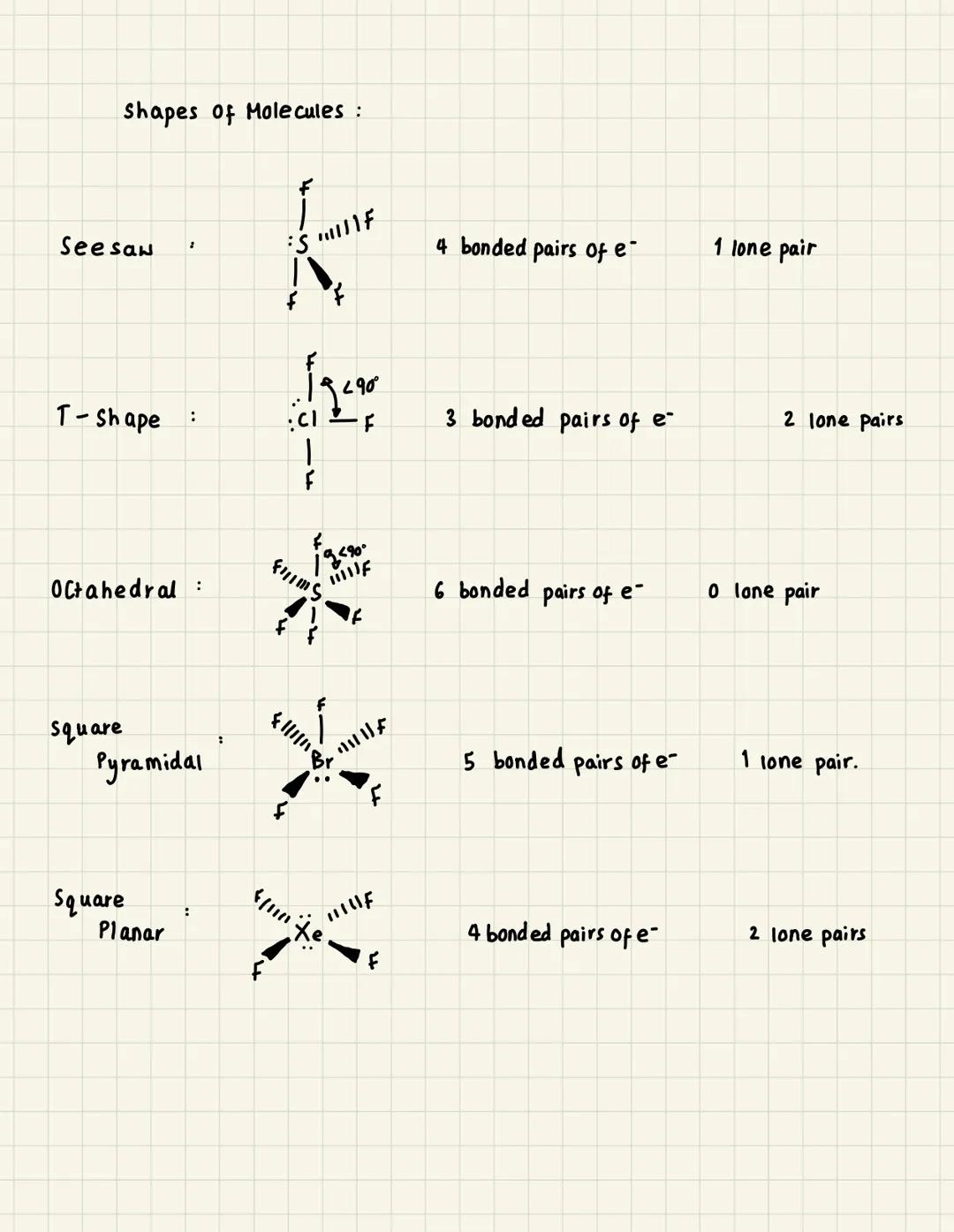
Basic Molecular Shapes
Linear molecules like carbon dioxide (CO₂) have just 2 bonded pairs of electrons around the central atom. These arrange themselves at 180° to minimise repulsion, creating a straight line structure.
Trigonal planar shapes form when you've got 3 bonded pairs and no lone pairs. Think of boron compounds where the bonds spread out at 120° angles, creating a flat triangular arrangement.
Tetrahedral is probably the most common shape you'll encounter - methane (CH₄) is the classic example. With 4 bonded pairs, the bonds arrange at 109.5° angles, forming a 3D pyramid shape.
Quick Tip: Remember that lone pairs of electrons take up more space than bonded pairs, so they push bonded pairs closer together and change bond angles.
When lone pairs get involved, things get interesting. Trigonal pyramidal (like ammonia NH₃) starts as tetrahedral but one position is occupied by a lone pair, creating a pyramid. Bent or non-linear molecules like water (H₂O) have 2 lone pairs pushing the bonded pairs down to about 104.5°.