Ever wondered how table salt forms at the atomic level?... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
137
•
12 Feb 2026
•
JJ
@jjstudymaster
Ever wondered how table salt forms at the atomic level?... Show more


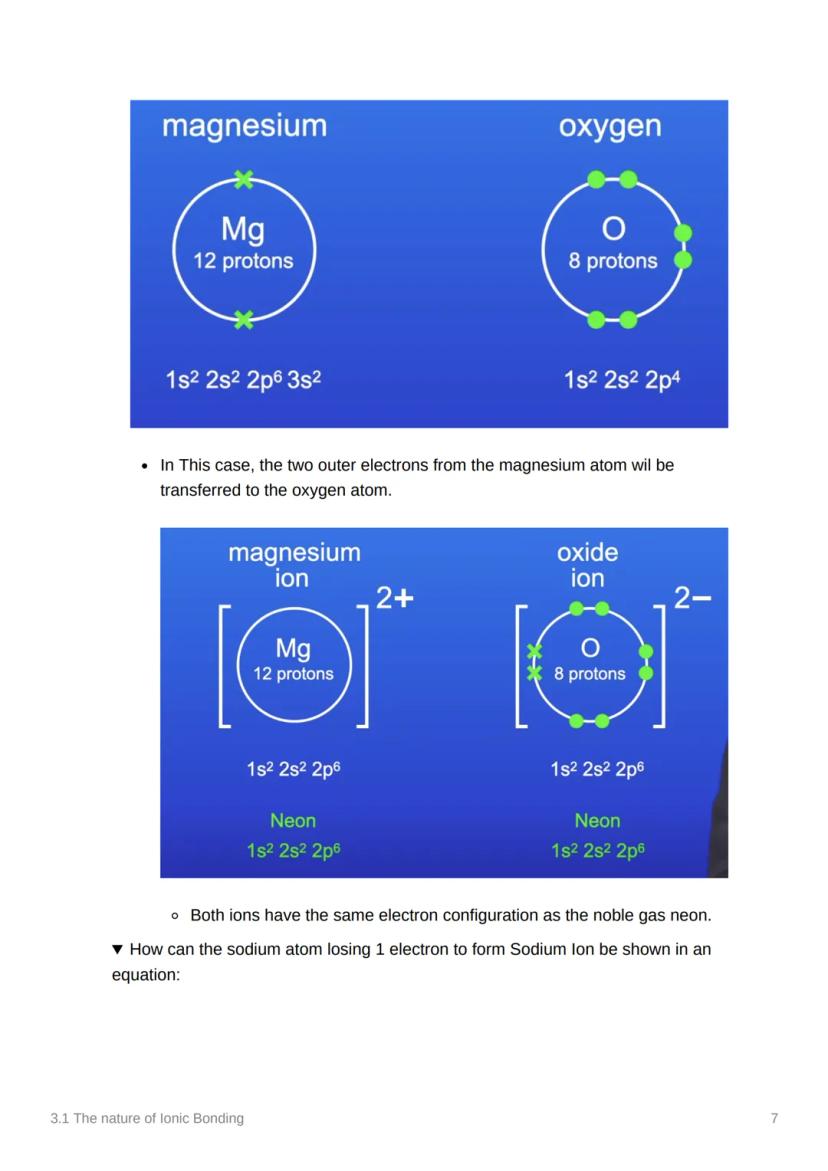
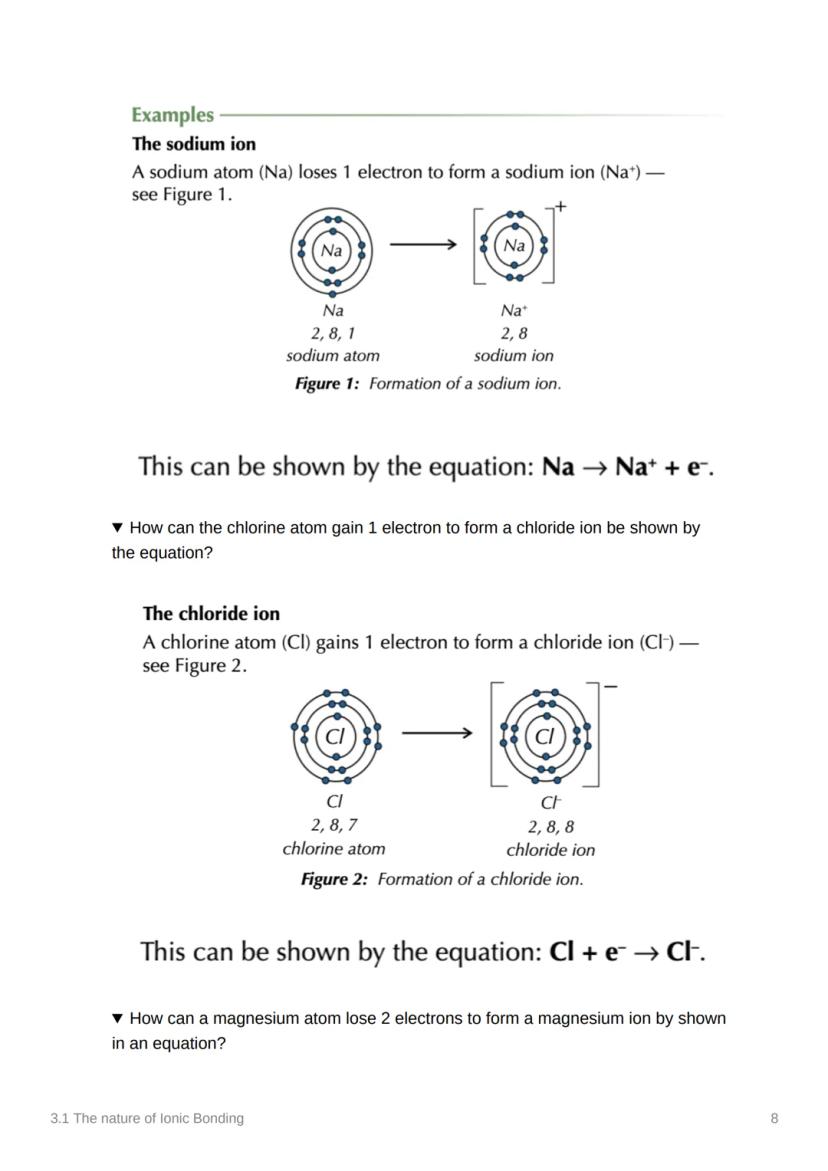
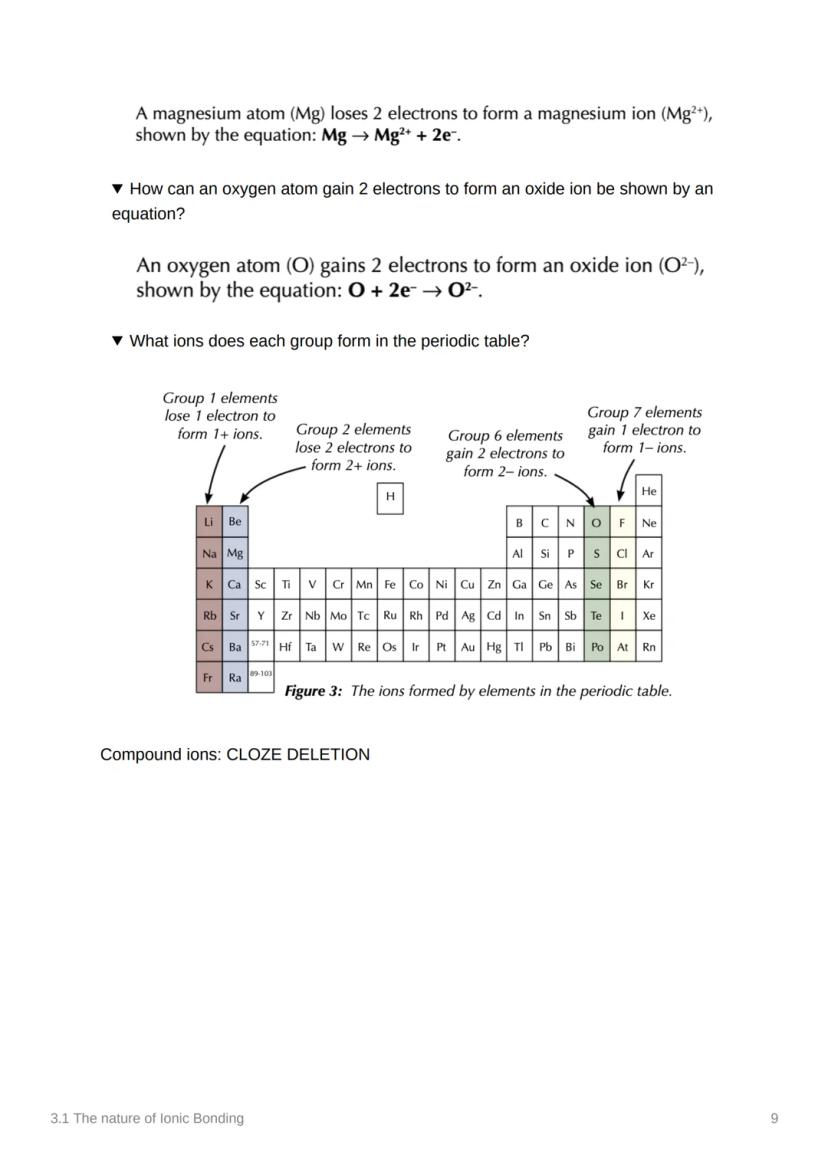
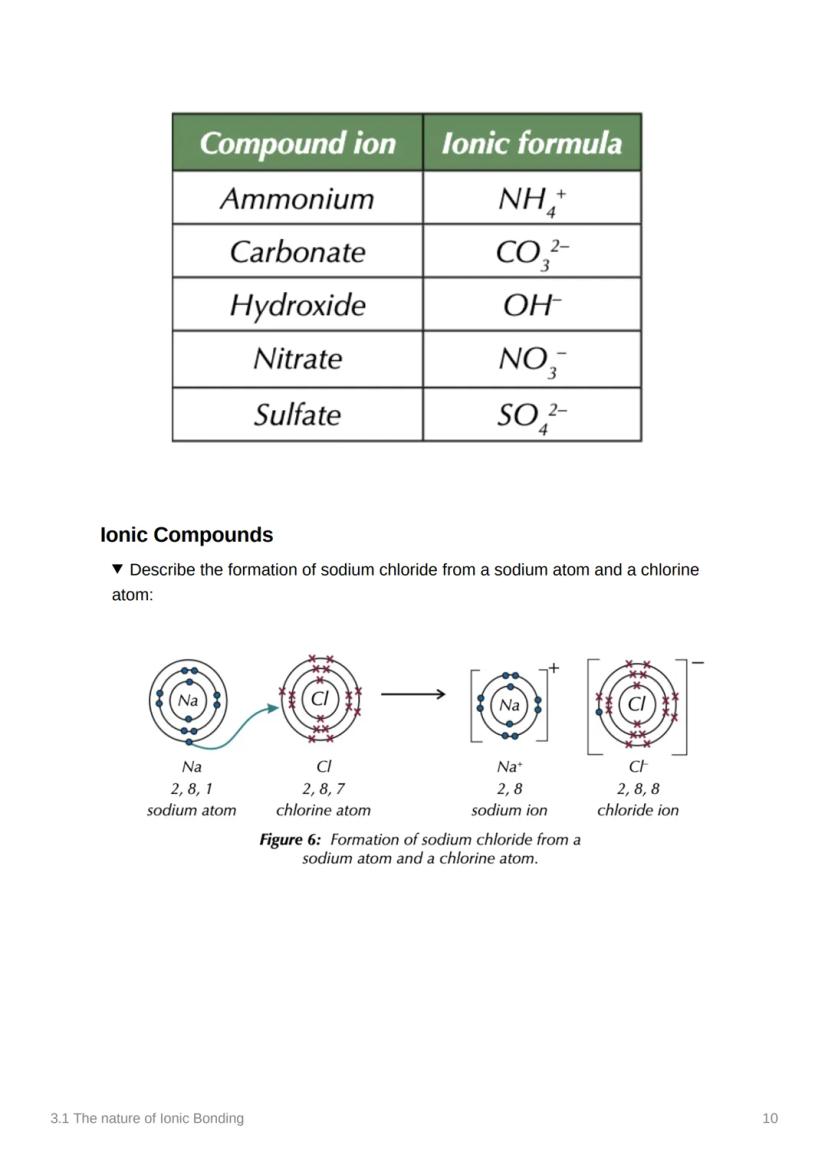
Atoms are basically trying to achieve the ultimate goal of stability - and they'll do whatever it takes to get there! Chemical bonds form when atoms share or transfer electrons to get a full outer energy level, just like the noble gases who are naturally stable.
There are three main types of strong chemical bonds you need to know: ionic, covalent, and metallic. Think of these as different strategies atoms use to achieve that coveted stable electron arrangement.
Key Insight: Atoms bond because they're seeking stability - it's like they're following nature's golden rule of achieving a full outer shell!
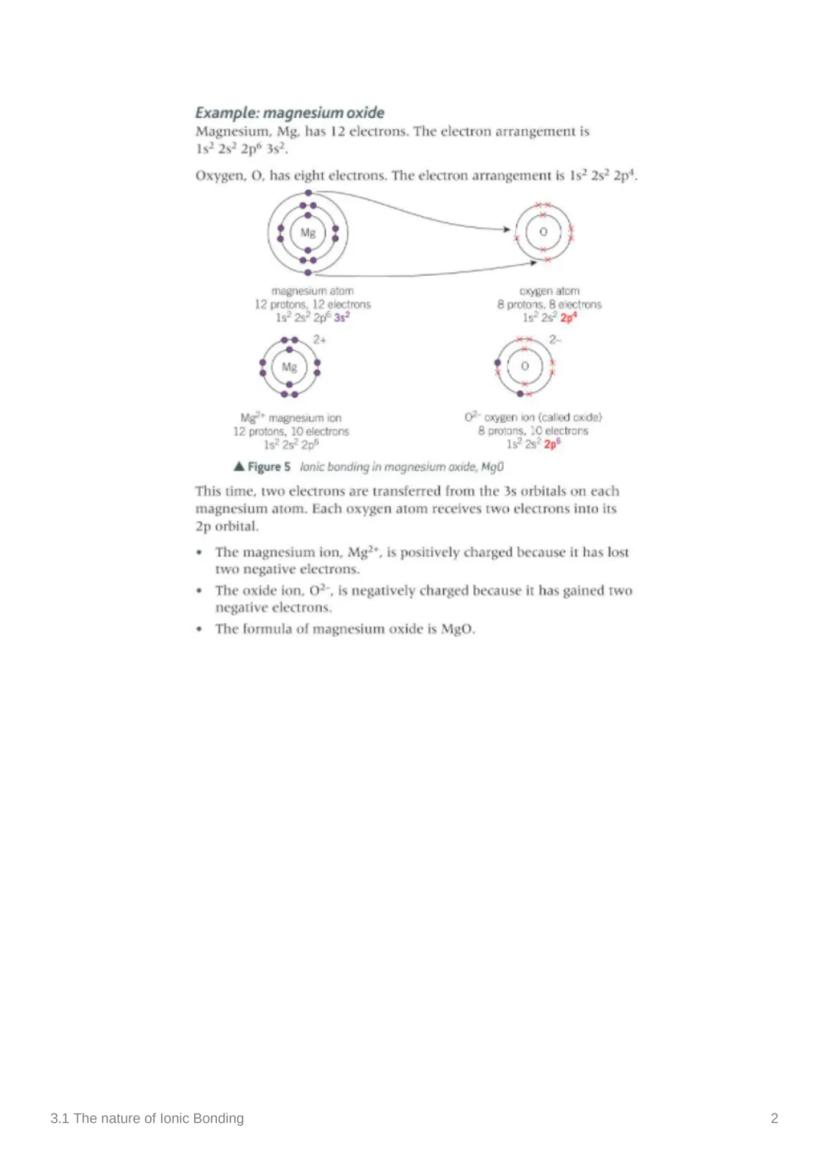
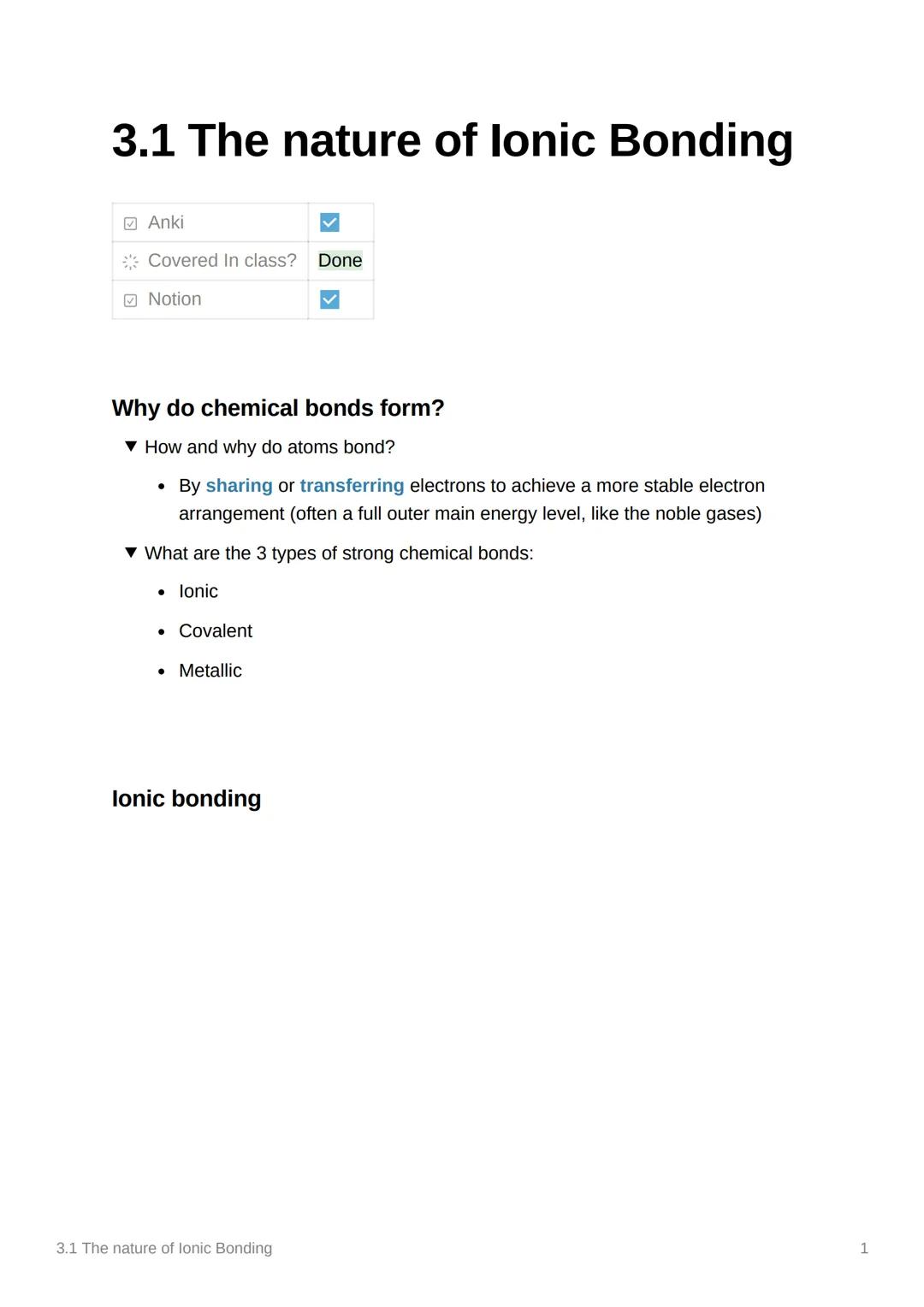
Let's see ionic bonding in action with magnesium oxide! Magnesium has 12 electrons (1s² 2s² 2p⁶ 3s²) whilst oxygen has 8 electrons (1s² 2s² 2p⁶). Here's where the magic happens.
Two electrons transfer from magnesium's 3s orbital to oxygen's 2p orbital. This creates Mg²⁺ (positively charged because it lost two negative electrons) and O²⁻ (negatively charged because it gained two electrons).
The result? MgO - magnesium oxide! Both ions now have stable electron arrangements, and the opposite charges create a powerful attraction that holds the compound together.
Remember: The number tells you how many electrons were transferred - Mg²⁺ lost 2, O²⁻ gained 2!
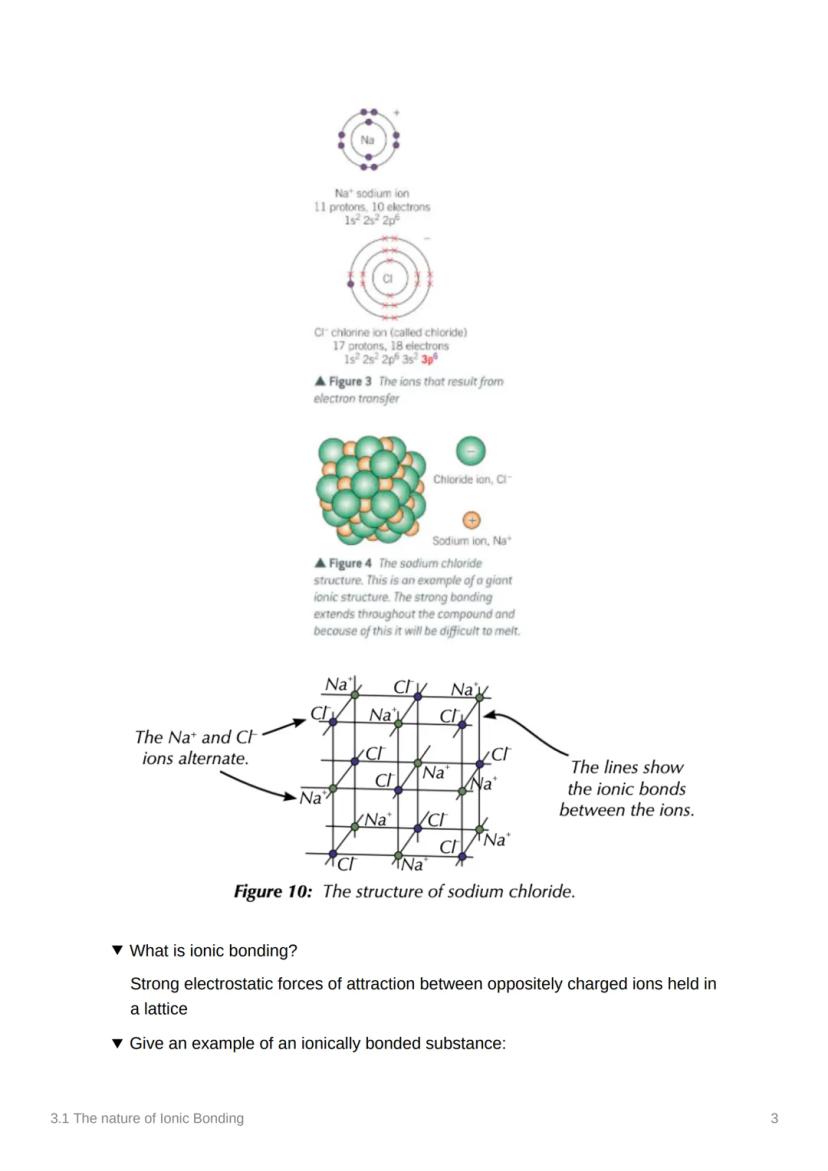
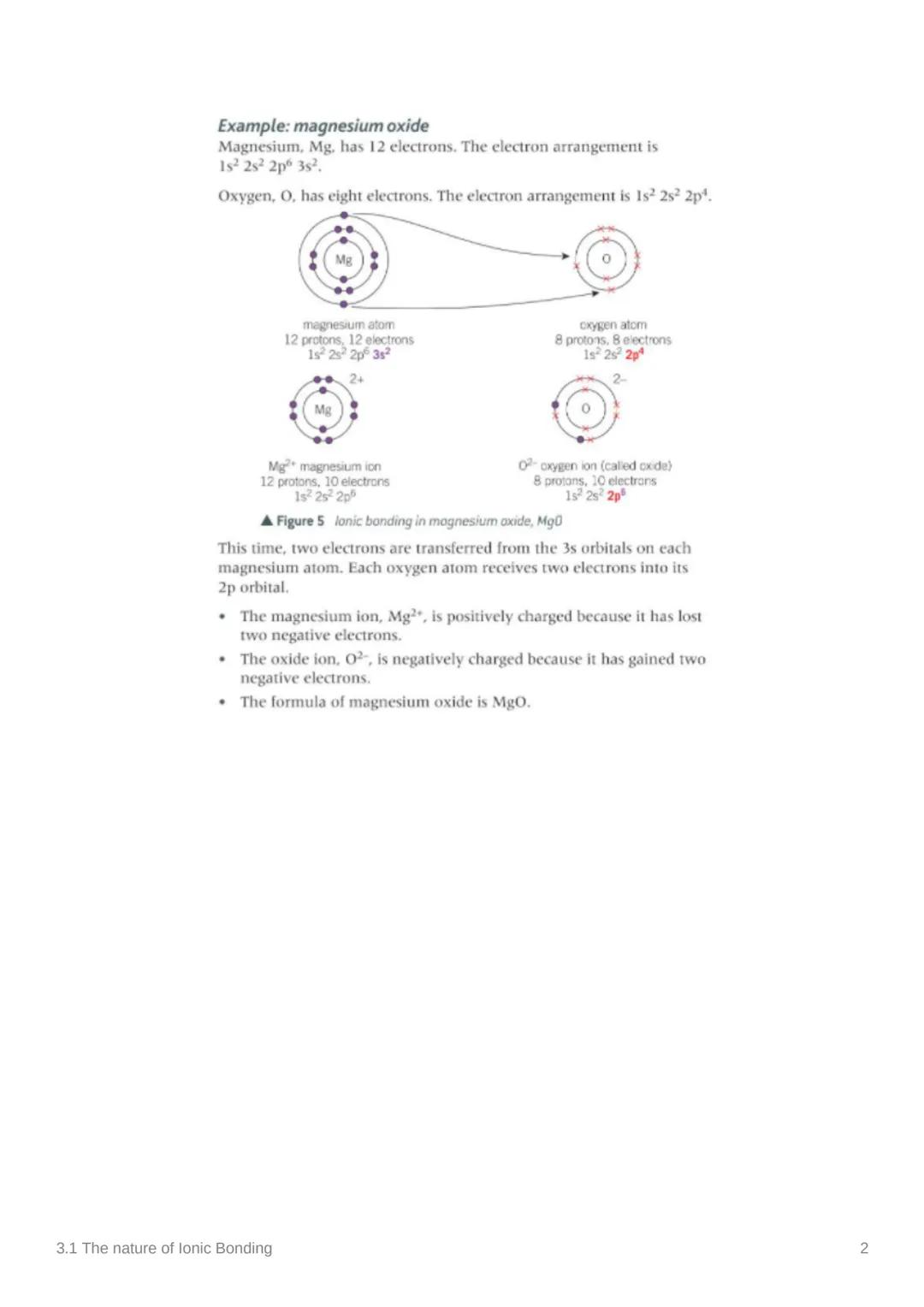
Sodium chloride isn't just two ions floating about - it forms a giant ionic structure where Na⁺ and Cl⁻ ions alternate in a repeating pattern. This creates an incredibly strong network held together by electrostatic forces.
Ionic bonding is defined as the strong electrostatic forces of attraction between oppositely charged ions held in a lattice. Think of it like a 3D puzzle where positive and negative pieces fit together perfectly.
This giant structure explains why salt is so difficult to melt - you're not just breaking apart two ions, you're disrupting an entire network of attractions!
Visual Tip: Picture ionic compounds as vast cities where positive and negative ions are neighbours, all holding hands through electrostatic attraction!

Here's the fundamental rule: ionic bonding occurs between metals and non-metals. Why? Because they have opposite needs! Metals need to lose electrons to achieve a full outer shell, whilst non-metals need to gain electrons.
The process is beautifully simple: the metal transfers its outer electrons to the non-metal. This creates oppositely charged ions that are then held together by electrostatic forces of attraction.
Sodium chloride (NaCl) is your classic example - sodium gives up one electron to chlorine, creating Na⁺ and Cl⁻ ions that stick together like magnets.
Memory Trick: Metals are generous (they give electrons), non-metals are greedy (they take electrons)!

When atoms lose electrons, they become positive ions (called cations). When they gain electrons, they become negative ions (called anions). It's all about the balance of protons and electrons!
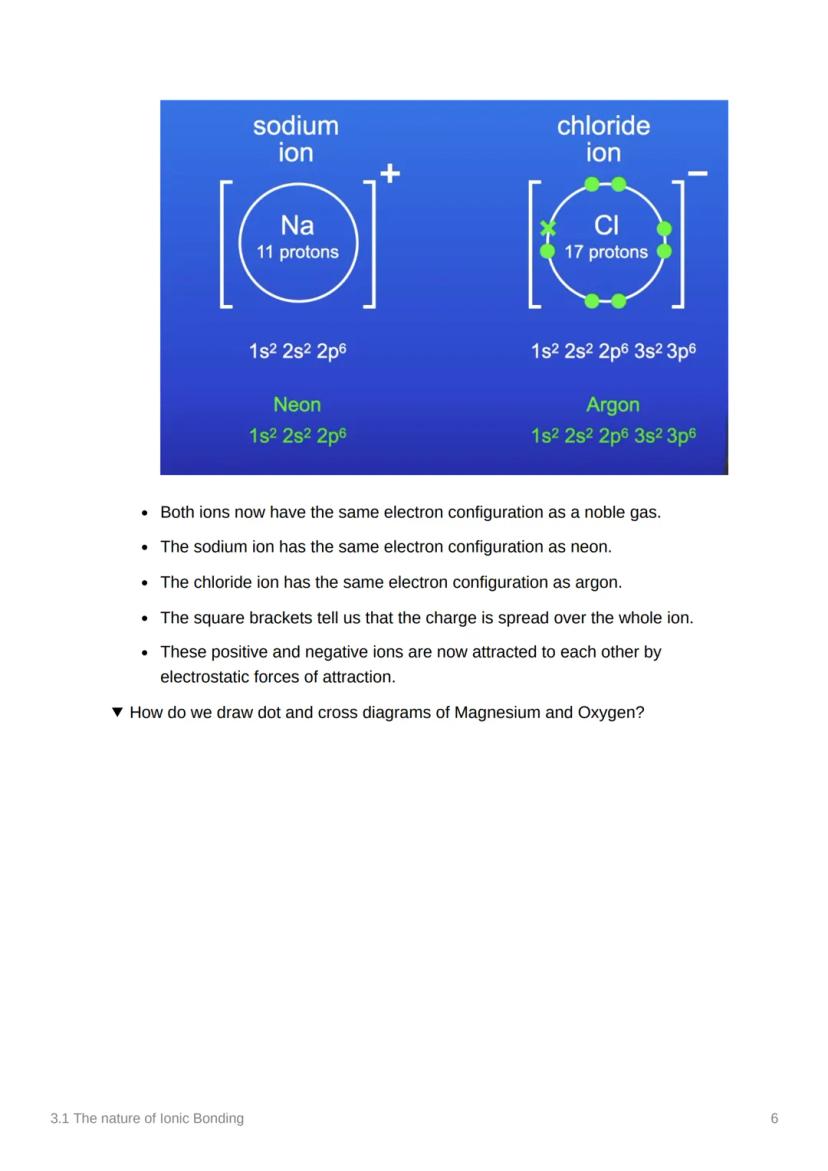
Let's look at sodium and chlorine: sodium (11 protons, 1s² 2s² 2p⁶ 3s¹) transfers its outer electron to chlorine (17 protons, 1s² 2s² 2p⁶ 3s² 3p⁵). This gives sodium the electron configuration of neon and chlorine the configuration of argon.
Dot and cross diagrams help visualise this transfer - you literally draw the electrons moving from one atom to another, showing how both achieve noble gas configurations.
Pro Tip: Count the protons and electrons in each ion to work out the charge - more protons than electrons = positive charge!
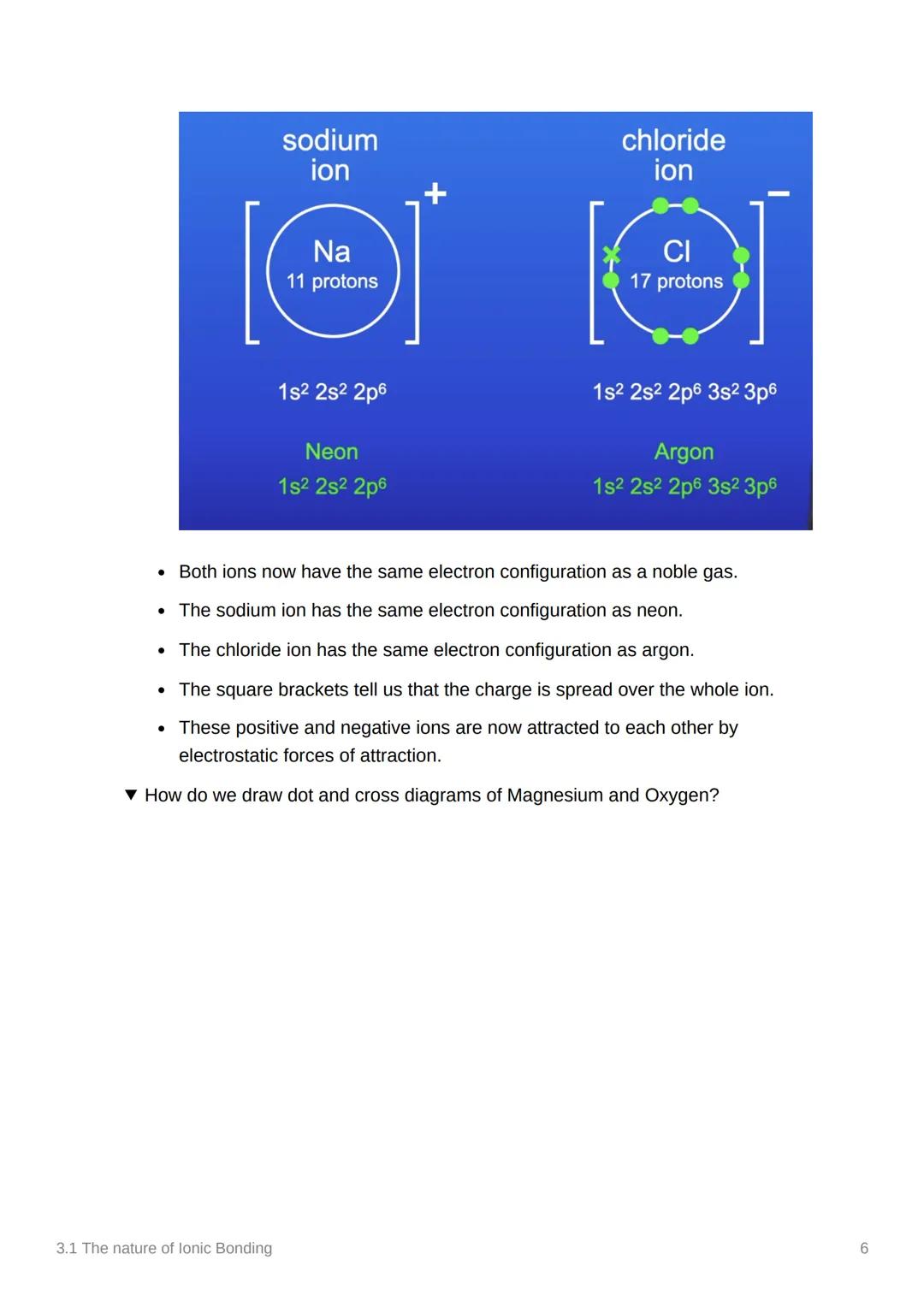
After electron transfer, both ions achieve the same electron configuration as noble gases - this is what makes them stable! The Na⁺ ion has neon's configuration (1s² 2s² 2p⁶) whilst Cl⁻ has argon's (1s² 2s² 2p⁶ 3s² 3p⁶).
The square brackets around the charge ([Na]⁺ and [Cl]⁻) show that the charge is spread over the entire ion, not just one part. These oppositely charged ions are now attracted to each other by powerful electrostatic forces.
This attraction is what creates the ionic bond - it's like the ions are permanently magnetised to each other!
Key Point: Achieving a noble gas configuration is the driving force behind all ionic bonding - atoms really want that stability!
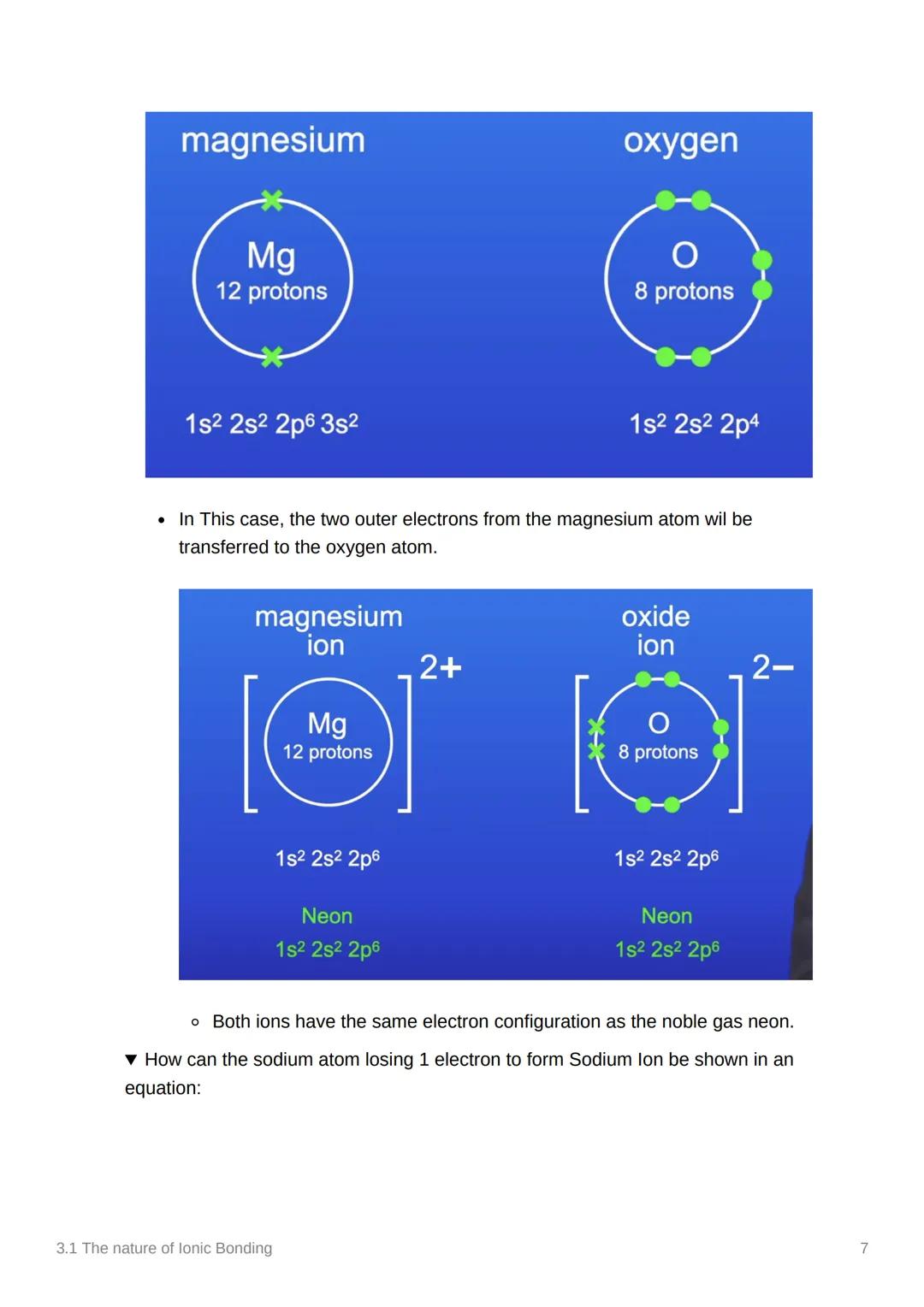
With magnesium and oxygen, we see a double electron transfer. Magnesium (12 protons, 1s² 2s² 2p⁶ 3s²) transfers both outer electrons to oxygen (8 protons, 1s² 2s² 2p⁴).
This creates Mg²⁺ and O²⁻ ions, both with the same electron configuration as neon (1s² 2s² 2p⁶). The charges are higher because more electrons were transferred, but the principle is identical.
The beauty of this system is that both ions end up with noble gas configurations, making them incredibly stable and strongly attracted to each other.
Pattern Recognition: Group 2 metals always lose 2 electrons, Group 6 non-metals always gain 2 electrons!
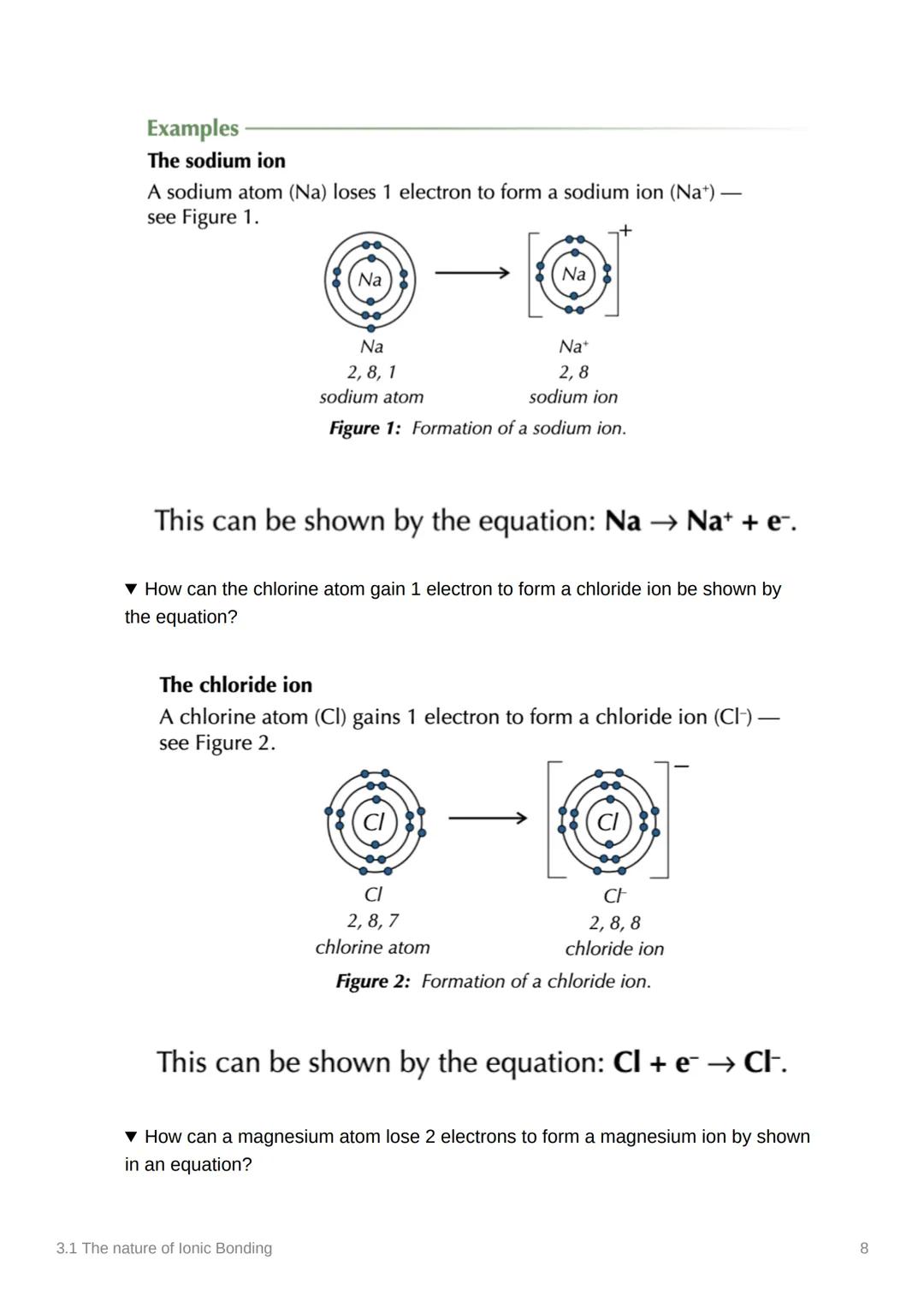
You can show electron transfer using simple equations. For sodium: Na → Na⁺ + e⁻ (loses one electron). For chlorine: Cl + e⁻ → Cl⁻ (gains one electron).
These equations are like chemical accounting - they show exactly what happens to each electron. The e⁻ represents the electron being lost or gained.
For magnesium: Mg → Mg²⁺ + 2e⁻ (loses two electrons). The number before e⁻ tells you how many electrons are involved in the transfer.
Equation Tip: The arrow shows the direction - atoms losing electrons have arrows pointing away from them, atoms gaining electrons have arrows pointing towards them!
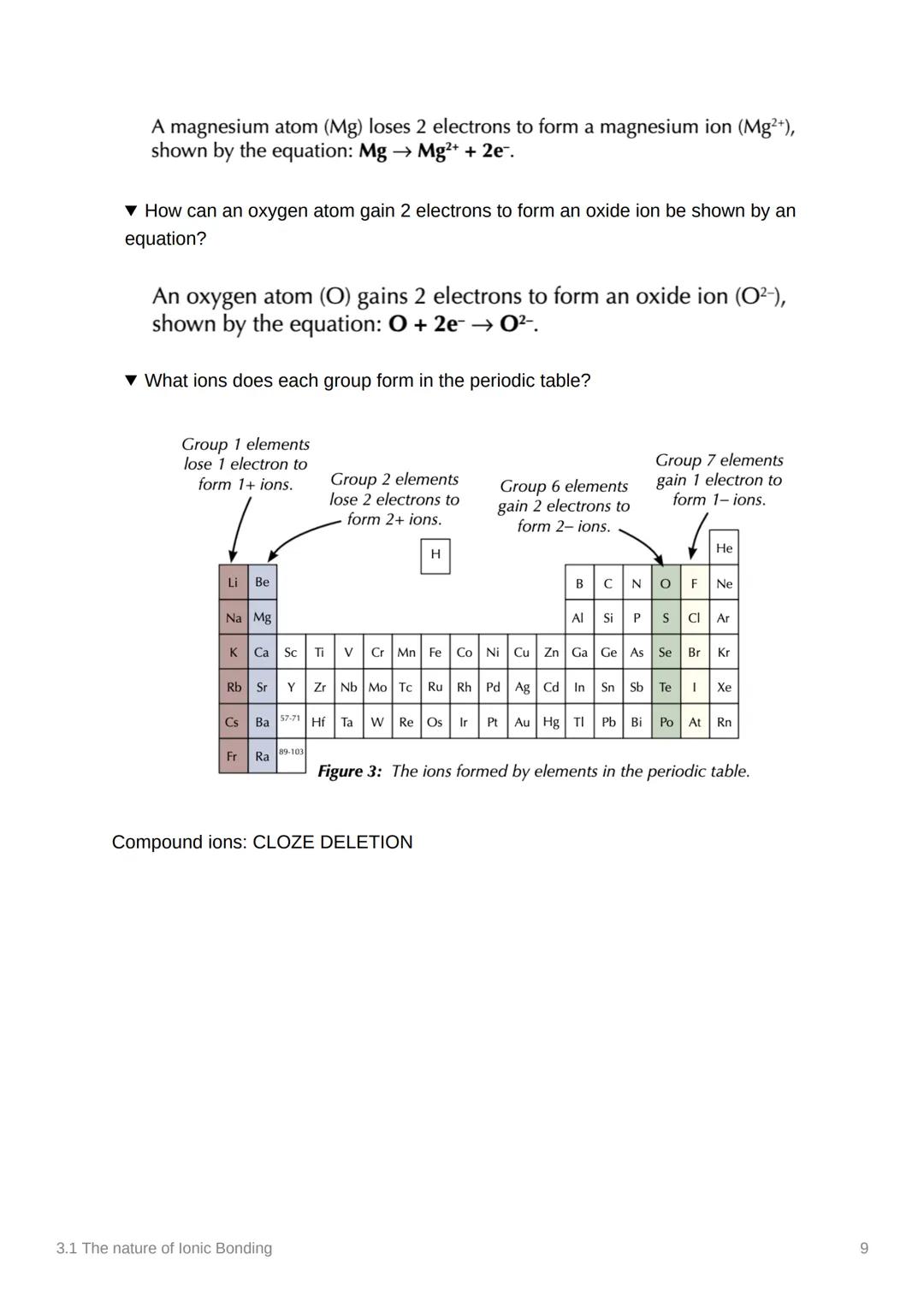
The periodic table gives you a roadmap for ionic charges! Group 1 elements lose 1 electron (1⁺ ions), Group 2 elements lose 2 electrons (2⁺ ions). On the other side, Group 6 elements gain 2 electrons (2⁻ ions) and Group 7 elements gain 1 electron (1⁻ ions).
For oxygen forming an oxide ion: O + 2e⁻ → O²⁻. The pattern is beautifully predictable once you know the groups!
Compound ions like ammonium (NH₄⁺), carbonate (CO₃²⁻), hydroxide (OH⁻), nitrate (NO₃⁻), and sulfate (SO₄²⁻) are special cases where groups of atoms act as single charged units.
Memory Aid: Groups 1 and 2 lose electrons (positive charges), Groups 6 and 7 gain electrons (negative charges) - it's all about getting to that magic number 8!
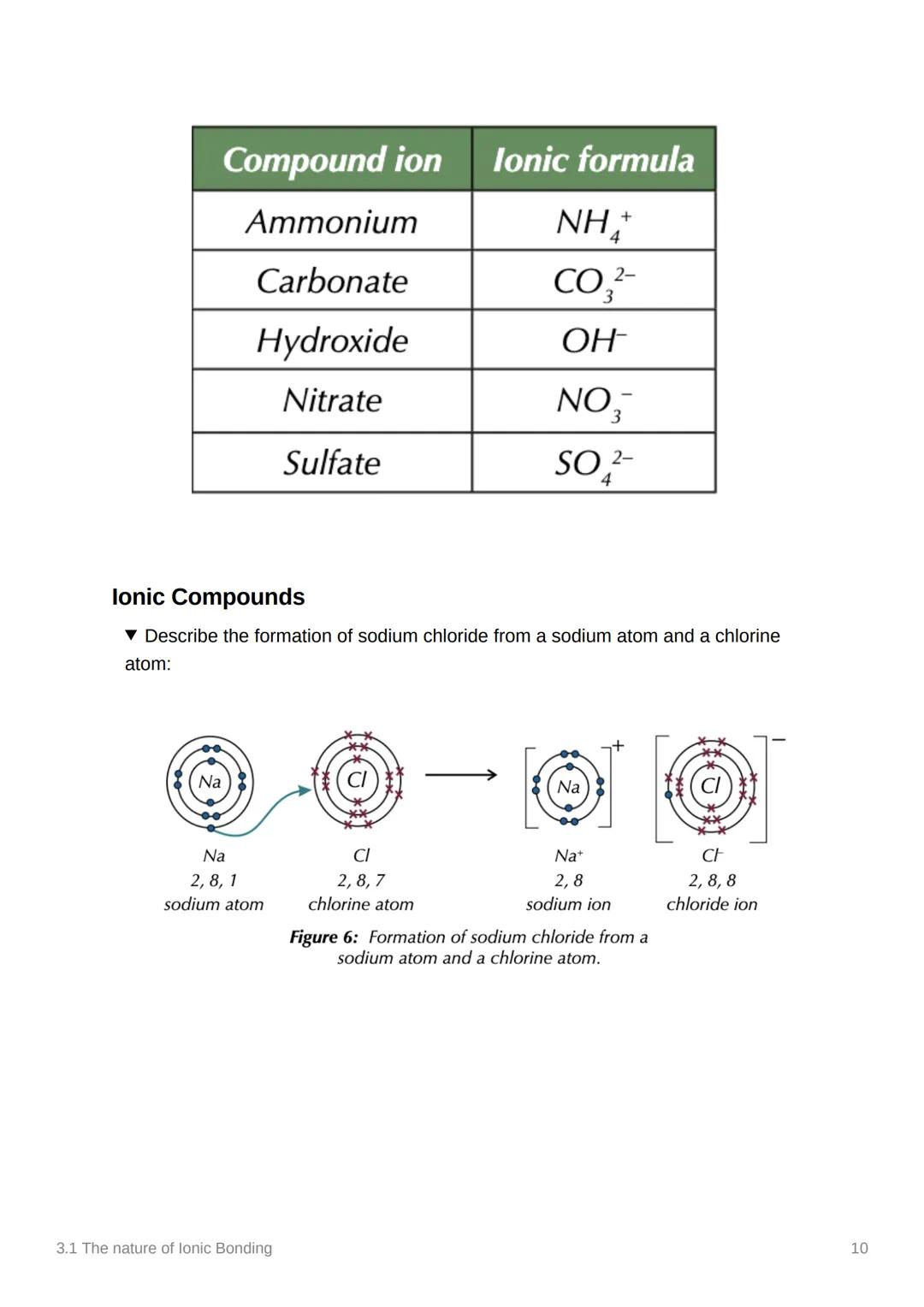
Let's put it all together with sodium chloride formation! A sodium atom (2,8,1) meets a chlorine atom (2,8,7) - it's like they're made for each other.
The sodium atom transfers one electron to the chlorine atom, creating Na⁺ (2,8) and Cl⁻ (2,8,8). Both ions now have stable electron arrangements and are held together by electrostatic attraction.
This process happens millions of times to create the giant ionic lattice we know as table salt. Every grain contains countless Na⁺ and Cl⁻ ions arranged in that perfect alternating pattern.
Real-World Connection: Every time you add salt to your chips, you're experiencing the power of ionic bonding holding those ions together!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
JJ
@jjstudymaster
Ever wondered how table salt forms at the atomic level? Ionic bonding is one of chemistry's fundamental forces that creates compounds when metals and non-metals get together to share electrons and achieve stability.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Atoms are basically trying to achieve the ultimate goal of stability - and they'll do whatever it takes to get there! Chemical bonds form when atoms share or transfer electrons to get a full outer energy level, just like the noble gases who are naturally stable.
There are three main types of strong chemical bonds you need to know: ionic, covalent, and metallic. Think of these as different strategies atoms use to achieve that coveted stable electron arrangement.
Key Insight: Atoms bond because they're seeking stability - it's like they're following nature's golden rule of achieving a full outer shell!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Let's see ionic bonding in action with magnesium oxide! Magnesium has 12 electrons (1s² 2s² 2p⁶ 3s²) whilst oxygen has 8 electrons (1s² 2s² 2p⁶). Here's where the magic happens.
Two electrons transfer from magnesium's 3s orbital to oxygen's 2p orbital. This creates Mg²⁺ (positively charged because it lost two negative electrons) and O²⁻ (negatively charged because it gained two electrons).
The result? MgO - magnesium oxide! Both ions now have stable electron arrangements, and the opposite charges create a powerful attraction that holds the compound together.
Remember: The number tells you how many electrons were transferred - Mg²⁺ lost 2, O²⁻ gained 2!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Sodium chloride isn't just two ions floating about - it forms a giant ionic structure where Na⁺ and Cl⁻ ions alternate in a repeating pattern. This creates an incredibly strong network held together by electrostatic forces.
Ionic bonding is defined as the strong electrostatic forces of attraction between oppositely charged ions held in a lattice. Think of it like a 3D puzzle where positive and negative pieces fit together perfectly.
This giant structure explains why salt is so difficult to melt - you're not just breaking apart two ions, you're disrupting an entire network of attractions!
Visual Tip: Picture ionic compounds as vast cities where positive and negative ions are neighbours, all holding hands through electrostatic attraction!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's the fundamental rule: ionic bonding occurs between metals and non-metals. Why? Because they have opposite needs! Metals need to lose electrons to achieve a full outer shell, whilst non-metals need to gain electrons.
The process is beautifully simple: the metal transfers its outer electrons to the non-metal. This creates oppositely charged ions that are then held together by electrostatic forces of attraction.
Sodium chloride (NaCl) is your classic example - sodium gives up one electron to chlorine, creating Na⁺ and Cl⁻ ions that stick together like magnets.
Memory Trick: Metals are generous (they give electrons), non-metals are greedy (they take electrons)!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When atoms lose electrons, they become positive ions (called cations). When they gain electrons, they become negative ions (called anions). It's all about the balance of protons and electrons!
Let's look at sodium and chlorine: sodium (11 protons, 1s² 2s² 2p⁶ 3s¹) transfers its outer electron to chlorine (17 protons, 1s² 2s² 2p⁶ 3s² 3p⁵). This gives sodium the electron configuration of neon and chlorine the configuration of argon.
Dot and cross diagrams help visualise this transfer - you literally draw the electrons moving from one atom to another, showing how both achieve noble gas configurations.
Pro Tip: Count the protons and electrons in each ion to work out the charge - more protons than electrons = positive charge!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
After electron transfer, both ions achieve the same electron configuration as noble gases - this is what makes them stable! The Na⁺ ion has neon's configuration (1s² 2s² 2p⁶) whilst Cl⁻ has argon's (1s² 2s² 2p⁶ 3s² 3p⁶).
The square brackets around the charge ([Na]⁺ and [Cl]⁻) show that the charge is spread over the entire ion, not just one part. These oppositely charged ions are now attracted to each other by powerful electrostatic forces.
This attraction is what creates the ionic bond - it's like the ions are permanently magnetised to each other!
Key Point: Achieving a noble gas configuration is the driving force behind all ionic bonding - atoms really want that stability!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
With magnesium and oxygen, we see a double electron transfer. Magnesium (12 protons, 1s² 2s² 2p⁶ 3s²) transfers both outer electrons to oxygen (8 protons, 1s² 2s² 2p⁴).
This creates Mg²⁺ and O²⁻ ions, both with the same electron configuration as neon (1s² 2s² 2p⁶). The charges are higher because more electrons were transferred, but the principle is identical.
The beauty of this system is that both ions end up with noble gas configurations, making them incredibly stable and strongly attracted to each other.
Pattern Recognition: Group 2 metals always lose 2 electrons, Group 6 non-metals always gain 2 electrons!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
You can show electron transfer using simple equations. For sodium: Na → Na⁺ + e⁻ (loses one electron). For chlorine: Cl + e⁻ → Cl⁻ (gains one electron).
These equations are like chemical accounting - they show exactly what happens to each electron. The e⁻ represents the electron being lost or gained.
For magnesium: Mg → Mg²⁺ + 2e⁻ (loses two electrons). The number before e⁻ tells you how many electrons are involved in the transfer.
Equation Tip: The arrow shows the direction - atoms losing electrons have arrows pointing away from them, atoms gaining electrons have arrows pointing towards them!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The periodic table gives you a roadmap for ionic charges! Group 1 elements lose 1 electron (1⁺ ions), Group 2 elements lose 2 electrons (2⁺ ions). On the other side, Group 6 elements gain 2 electrons (2⁻ ions) and Group 7 elements gain 1 electron (1⁻ ions).
For oxygen forming an oxide ion: O + 2e⁻ → O²⁻. The pattern is beautifully predictable once you know the groups!
Compound ions like ammonium (NH₄⁺), carbonate (CO₃²⁻), hydroxide (OH⁻), nitrate (NO₃⁻), and sulfate (SO₄²⁻) are special cases where groups of atoms act as single charged units.
Memory Aid: Groups 1 and 2 lose electrons (positive charges), Groups 6 and 7 gain electrons (negative charges) - it's all about getting to that magic number 8!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Let's put it all together with sodium chloride formation! A sodium atom (2,8,1) meets a chlorine atom (2,8,7) - it's like they're made for each other.
The sodium atom transfers one electron to the chlorine atom, creating Na⁺ (2,8) and Cl⁻ (2,8,8). Both ions now have stable electron arrangements and are held together by electrostatic attraction.
This process happens millions of times to create the giant ionic lattice we know as table salt. Every grain contains countless Na⁺ and Cl⁻ ions arranged in that perfect alternating pattern.
Real-World Connection: Every time you add salt to your chips, you're experiencing the power of ionic bonding holding those ions together!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
6
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the fundamentals of chemical bonding, including covalent and ionic bonds, molecular shapes, hybridization, and intermolecular forces. This comprehensive summary covers key concepts such as bond polarity, resonance structures, and the octet rule, essential for mastering IB Chemistry HL. Ideal for exam preparation and understanding complex chemical interactions.
Explore key concepts in bonding and electronegativity, including trends across periods and groups, types of bonds (ionic, covalent, polar), and molecular shapes. This summary covers essential topics for OCR A Level Chemistry, providing insights into intermolecular forces, bond polarity, and the impact of electronegativity on molecular behavior.
Explore key trends in the periodic table, focusing on atomic radius, ionization energy, and melting points. This summary highlights how these properties change across periods and groups, including the impact of atomic structure and intermolecular forces. Ideal for A-level chemistry students seeking to understand periodic trends.
Explore the key concepts of periodicity in chemistry, including ionization energy, electronegativity, atomic radius, and covalent bonding. This summary covers the periodic trends across groups and periods, detailing the properties of covalent network solids and their structures. Ideal for SQA Higher Chemistry students seeking a comprehensive understanding of the periodic table and its implications.
Explore key periodic trends in Higher Chemistry, including ionization energy, electronegativity, and covalent radius. This summary provides essential insights into how these properties change across periods and down groups, with a focus on their implications in chemical behavior. Ideal for students preparing for exams in Unit 1: Chemical Changes and Structure.
Explore the intricacies of chemical bonding, including covalent and ionic interactions, molecular shapes, and bond angles. This summary covers key concepts such as tetrahedral and octahedral geometries, lone pairs, and the effects of molecular structure on properties. Ideal for A-level chemistry students seeking to understand the fundamentals of molecular geometry and bonding forces.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user