Ever wondered what atoms are actually made of and how... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
157
•
9 Feb 2026
•
Posy Chapman
@osyhapman_okhwsrybqi
Ever wondered what atoms are actually made of and how... Show more








Your understanding of atoms has come a long way since John Dalton's solid sphere model in 1803. Scientists like J.J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrödinger each added pieces to the puzzle, leading to our current quantum model.
Every atom contains three fundamental particles with specific properties you need to memorise. Protons have a relative mass of 1 and charge of +1, neutrons have a relative mass of 1 and no charge, whilst electrons have a tiny relative mass of 1/1836 and charge of -1.
The nucleus sits at the atom's centre, containing all protons and neutrons (called nucleons). Since electrons are so light, virtually all the atom's mass concentrates in this tiny nucleus.
Quick tip: Remember that electrons are about 1,800 times lighter than protons and neutrons - they barely contribute to the atom's mass!

Working out particle numbers is straightforward once you know the rules. The atomic number equals the number of protons, whilst the mass number equals protons plus neutrons combined. In neutral atoms, electrons equal protons, but in ions, you subtract the charge from the atomic number.
Isotopes are atoms of the same element with different numbers of neutrons. For example, carbon-12, carbon-13, and carbon-14 all have 6 protons but different neutrons. These isotopes behave identically in chemical reactions because they have the same electron arrangement.
Scientists use mass spectrometers to study isotopes and calculate relative atomic mass. This average mass accounts for all isotopes and their abundance in nature.
Exam focus: You'll often need to calculate particle numbers from mass and atomic numbers - practice this skill until it becomes automatic!

Time of flight (TOF) mass spectrometry works by giving all ions the same kinetic energy, then measuring how fast they travel. Since kinetic energy equals ½mv², lighter ions zoom through faster than heavier ones.
The process involves five key steps: vaporising the sample, ionisation, acceleration in an electric field, separation in the flight tube, and detection. The detector records flight times and converts them into a mass spectrum showing relative abundance against mass-to-charge ratio.
Scientists can use two ionisation methods. Electron impact fires high-energy electrons at the sample, whilst electrospray ionisation uses charged droplets from a volatile solvent. Electron impact can fragment larger molecules, so electrospray works better for delicate compounds.
Real-world connection: Mass spectrometry helps identify unknown substances in everything from forensic investigations to pharmaceutical development!

Electron impact ionisation works by bombarding vaporised samples with high-energy electrons at low pressure. This knocks off outer electrons, creating positive ions. However, this method can fragment larger organic molecules due to its high energy.
Electrospray ionisation offers a gentler approach. The sample dissolves in a polar, volatile solvent and gets pumped through a narrow capillary tube. A high voltage creates charged droplets, and as the solvent evaporates, gaseous ions form.
After ionisation, an electric field accelerates all ions to the same kinetic energy. In the flight tube, lighter ions travel faster and reach the detector first, allowing separation by mass.
Key insight: Choose electron impact for small molecules and elements, but use electrospray for larger, fragile organic compounds to prevent fragmentation!

The detector records ion flight times and measures abundance through the electric current produced when positive ions arrive. Faster, lighter ions create signals first, followed by slower, heavier ones.
Data analysis converts flight times into a mass spectrum - a graph plotting relative abundance against mass-to-charge ratio . This spectrum acts like a fingerprint, helping identify unknown substances and determine molecular masses.
Understanding key terms helps: polar molecules have positive and negative ends, volatile solvents evaporate easily, and capillary tubes are incredibly thin tubes that move liquids against gravity. Abundance simply means the percentage of each isotope present.
Practical tip: When calculating relative atomic mass from abundance data, remember all percentages must add up to 100% - use this to find unknown values!

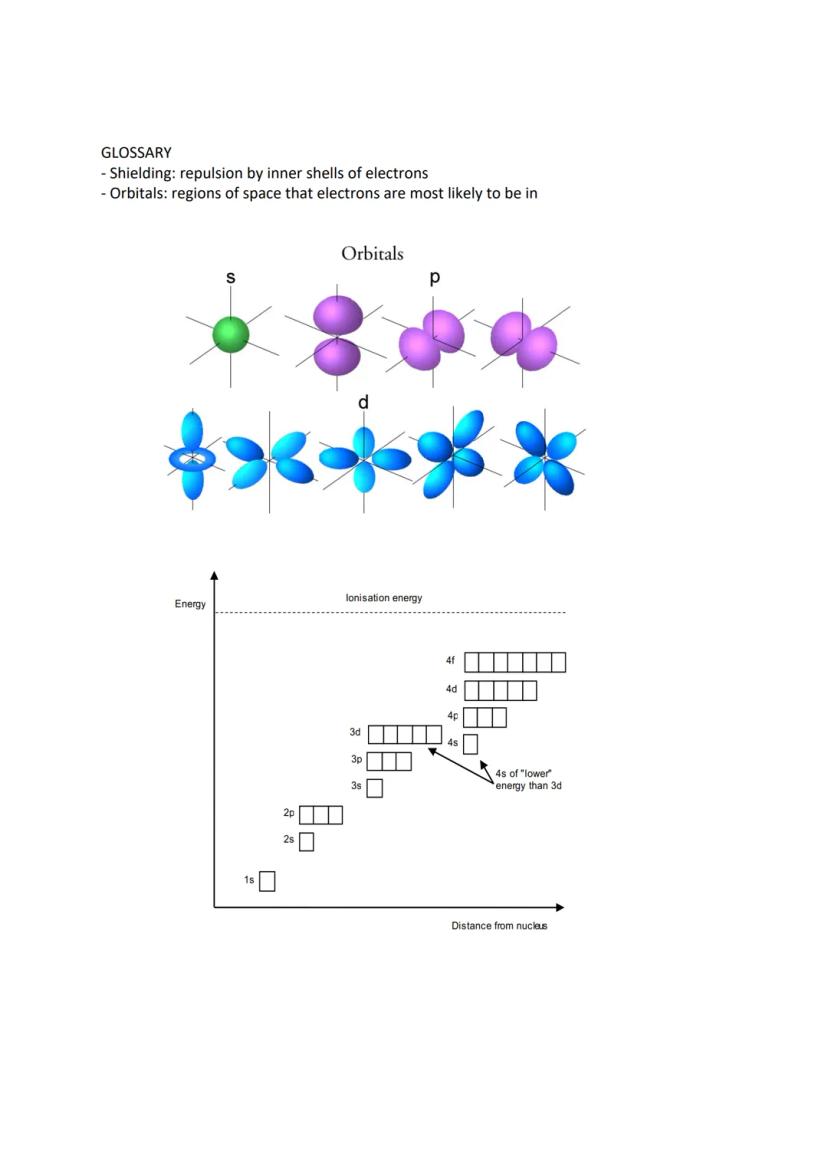
Electrons don't just randomly orbit the nucleus - they occupy specific shells and sub-shells with particular shapes and energies. The first shell contains one s orbital, the second has one s and three p orbitals, and the third adds five d orbitals.
Each orbital holds maximum two electrons spinning in opposite directions. Hund's rule states that electrons prefer sitting alone in orbitals before pairing up, like people preferring empty bus seats.
There's a crucial exception: chromium and copper don't follow the expected pattern. Chromium has the configuration [Ar] 4s¹ 3d⁵ rather than 4s² 3d⁴, whilst copper has [Ar] 4s¹ 3d¹⁰ instead of 4s² 3d⁹. When forming ions, electrons are removed from the highest energy level first (4s before 3d).
Memory trick: Remember "4s before 3d" for both filling and emptying - it's counterintuitive but absolutely essential for A-level success!
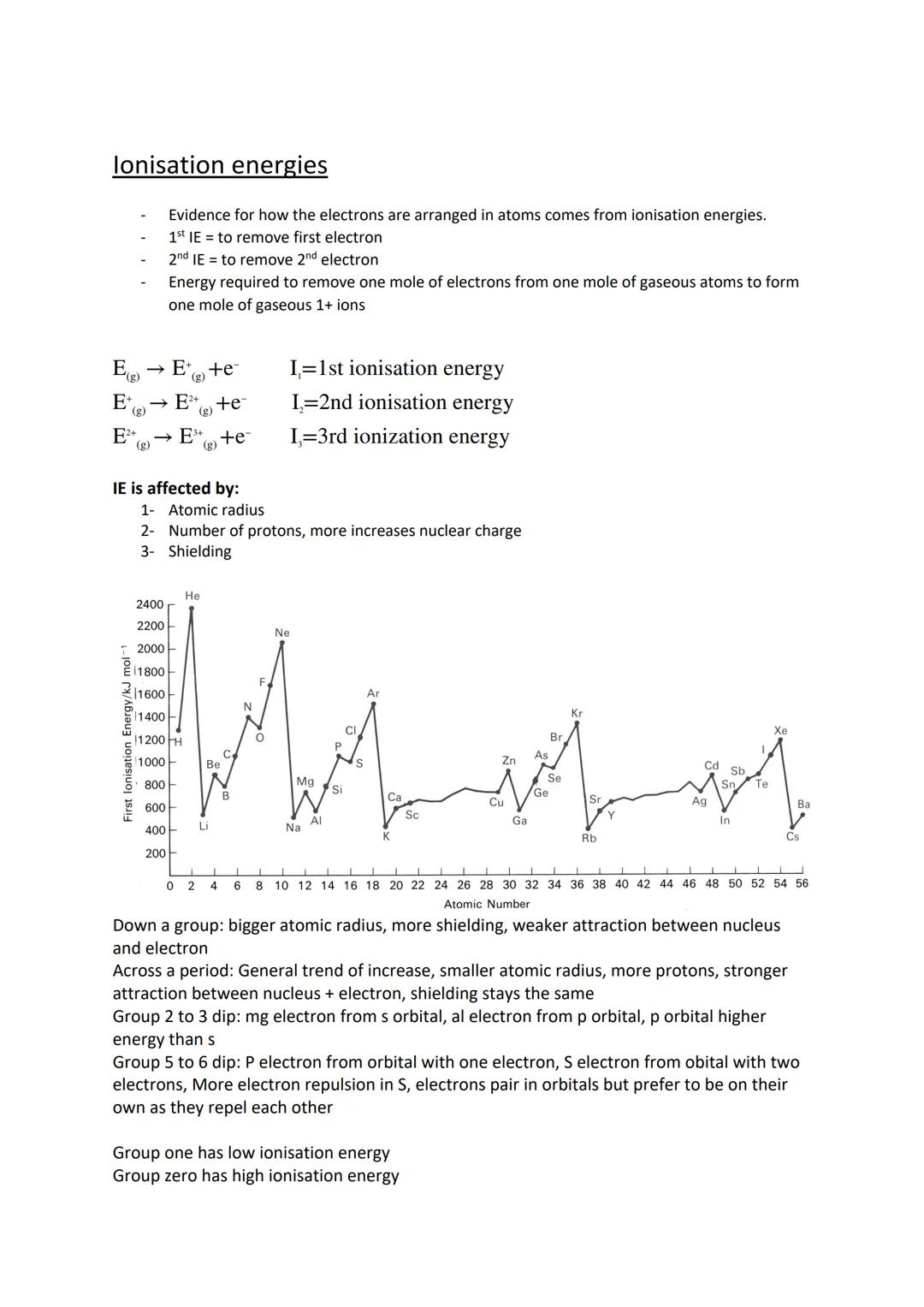
Ionisation energy measures the energy needed to remove electrons from gaseous atoms. The first ionisation energy removes one electron, the second removes another, and so on. Each successive ionisation energy increases because you're removing electrons from increasingly positive ions.
Three factors affect ionisation energy: atomic radius (larger atoms hold electrons less tightly), nuclear charge (more protons pull electrons stronger), and shielding (inner electrons repel outer ones).
Across periods, ionisation energy generally increases due to stronger nuclear attraction. However, there are two important dips: Group 2 to 3 (removing p electrons is easier than s electrons) and Group 5 to 6 (paired electrons in orbitals repel each other).
Pattern recognition: Group 1 elements have low ionisation energies (easy to lose electrons), whilst Group 0 elements have high values (very stable configurations)!
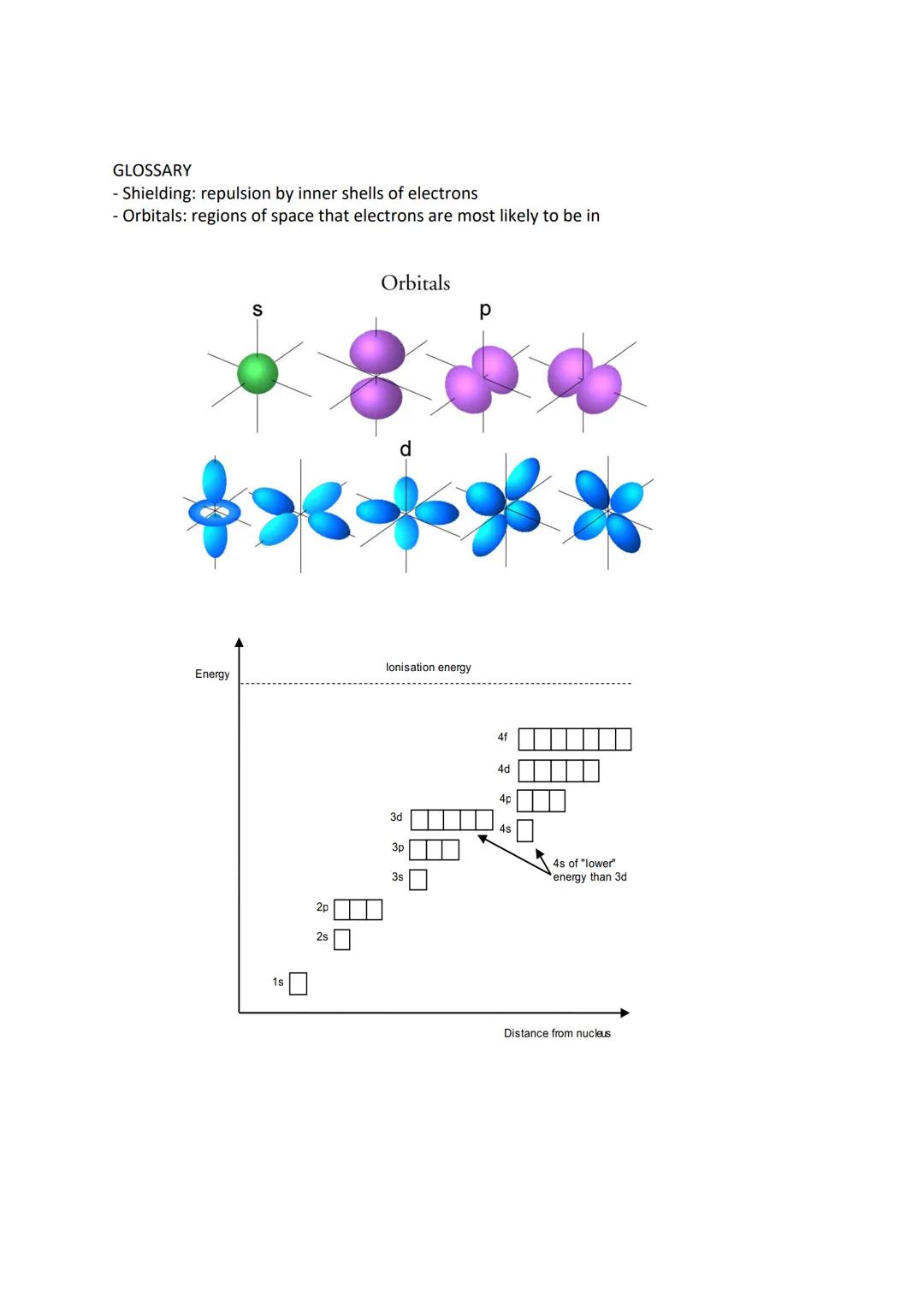
Understanding orbital shapes helps visualise electron arrangements. S orbitals are spherical, p orbitals are dumbbell-shaped, and d orbitals have more complex geometries. Energy increases as you move further from the nucleus.
Shielding occurs when inner electron shells repel outer electrons, reducing the nuclear attraction they feel. This explains why atoms get larger down groups despite having more protons.
Remember that 4s orbitals have lower energy than 3d orbitals when filling, but 4s electrons are removed first when forming ions. This seemingly contradictory behaviour is crucial for understanding transition metal chemistry.
Visual learning: Drawing orbital diagrams and electron configurations repeatedly will make these concepts stick - practice with different elements until it becomes second nature!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Posy Chapman
@osyhapman_okhwsrybqi
Ever wondered what atoms are actually made of and how we figured it out? Understanding atomic structure is like solving a massive puzzle that scientists have been working on for over 200 years, and it's absolutely crucial for your chemistry... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Your understanding of atoms has come a long way since John Dalton's solid sphere model in 1803. Scientists like J.J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrödinger each added pieces to the puzzle, leading to our current quantum model.
Every atom contains three fundamental particles with specific properties you need to memorise. Protons have a relative mass of 1 and charge of +1, neutrons have a relative mass of 1 and no charge, whilst electrons have a tiny relative mass of 1/1836 and charge of -1.
The nucleus sits at the atom's centre, containing all protons and neutrons (called nucleons). Since electrons are so light, virtually all the atom's mass concentrates in this tiny nucleus.
Quick tip: Remember that electrons are about 1,800 times lighter than protons and neutrons - they barely contribute to the atom's mass!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Working out particle numbers is straightforward once you know the rules. The atomic number equals the number of protons, whilst the mass number equals protons plus neutrons combined. In neutral atoms, electrons equal protons, but in ions, you subtract the charge from the atomic number.
Isotopes are atoms of the same element with different numbers of neutrons. For example, carbon-12, carbon-13, and carbon-14 all have 6 protons but different neutrons. These isotopes behave identically in chemical reactions because they have the same electron arrangement.
Scientists use mass spectrometers to study isotopes and calculate relative atomic mass. This average mass accounts for all isotopes and their abundance in nature.
Exam focus: You'll often need to calculate particle numbers from mass and atomic numbers - practice this skill until it becomes automatic!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Time of flight (TOF) mass spectrometry works by giving all ions the same kinetic energy, then measuring how fast they travel. Since kinetic energy equals ½mv², lighter ions zoom through faster than heavier ones.
The process involves five key steps: vaporising the sample, ionisation, acceleration in an electric field, separation in the flight tube, and detection. The detector records flight times and converts them into a mass spectrum showing relative abundance against mass-to-charge ratio.
Scientists can use two ionisation methods. Electron impact fires high-energy electrons at the sample, whilst electrospray ionisation uses charged droplets from a volatile solvent. Electron impact can fragment larger molecules, so electrospray works better for delicate compounds.
Real-world connection: Mass spectrometry helps identify unknown substances in everything from forensic investigations to pharmaceutical development!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electron impact ionisation works by bombarding vaporised samples with high-energy electrons at low pressure. This knocks off outer electrons, creating positive ions. However, this method can fragment larger organic molecules due to its high energy.
Electrospray ionisation offers a gentler approach. The sample dissolves in a polar, volatile solvent and gets pumped through a narrow capillary tube. A high voltage creates charged droplets, and as the solvent evaporates, gaseous ions form.
After ionisation, an electric field accelerates all ions to the same kinetic energy. In the flight tube, lighter ions travel faster and reach the detector first, allowing separation by mass.
Key insight: Choose electron impact for small molecules and elements, but use electrospray for larger, fragile organic compounds to prevent fragmentation!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The detector records ion flight times and measures abundance through the electric current produced when positive ions arrive. Faster, lighter ions create signals first, followed by slower, heavier ones.
Data analysis converts flight times into a mass spectrum - a graph plotting relative abundance against mass-to-charge ratio . This spectrum acts like a fingerprint, helping identify unknown substances and determine molecular masses.
Understanding key terms helps: polar molecules have positive and negative ends, volatile solvents evaporate easily, and capillary tubes are incredibly thin tubes that move liquids against gravity. Abundance simply means the percentage of each isotope present.
Practical tip: When calculating relative atomic mass from abundance data, remember all percentages must add up to 100% - use this to find unknown values!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrons don't just randomly orbit the nucleus - they occupy specific shells and sub-shells with particular shapes and energies. The first shell contains one s orbital, the second has one s and three p orbitals, and the third adds five d orbitals.
Each orbital holds maximum two electrons spinning in opposite directions. Hund's rule states that electrons prefer sitting alone in orbitals before pairing up, like people preferring empty bus seats.
There's a crucial exception: chromium and copper don't follow the expected pattern. Chromium has the configuration [Ar] 4s¹ 3d⁵ rather than 4s² 3d⁴, whilst copper has [Ar] 4s¹ 3d¹⁰ instead of 4s² 3d⁹. When forming ions, electrons are removed from the highest energy level first (4s before 3d).
Memory trick: Remember "4s before 3d" for both filling and emptying - it's counterintuitive but absolutely essential for A-level success!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ionisation energy measures the energy needed to remove electrons from gaseous atoms. The first ionisation energy removes one electron, the second removes another, and so on. Each successive ionisation energy increases because you're removing electrons from increasingly positive ions.
Three factors affect ionisation energy: atomic radius (larger atoms hold electrons less tightly), nuclear charge (more protons pull electrons stronger), and shielding (inner electrons repel outer ones).
Across periods, ionisation energy generally increases due to stronger nuclear attraction. However, there are two important dips: Group 2 to 3 (removing p electrons is easier than s electrons) and Group 5 to 6 (paired electrons in orbitals repel each other).
Pattern recognition: Group 1 elements have low ionisation energies (easy to lose electrons), whilst Group 0 elements have high values (very stable configurations)!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Understanding orbital shapes helps visualise electron arrangements. S orbitals are spherical, p orbitals are dumbbell-shaped, and d orbitals have more complex geometries. Energy increases as you move further from the nucleus.
Shielding occurs when inner electron shells repel outer electrons, reducing the nuclear attraction they feel. This explains why atoms get larger down groups despite having more protons.
Remember that 4s orbitals have lower energy than 3d orbitals when filling, but 4s electrons are removed first when forming ions. This seemingly contradictory behaviour is crucial for understanding transition metal chemistry.
Visual learning: Drawing orbital diagrams and electron configurations repeatedly will make these concepts stick - practice with different elements until it becomes second nature!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
0
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore key concepts in atomic structure and periodic trends, including ionization energy, electron configuration, mass spectrometry, and the electromagnetic spectrum. This summary covers essential topics for A Level Chemistry, focusing on the periodic table, isotopes, and quantum energy levels. Ideal for Edexcel students preparing for exams.
Explore the concepts of atom economy, percentage yield, and the significance of hydrated vs. anhydrous salts in A-level Physical Chemistry. This summary covers key calculations, the ideal gas equation, and the importance of efficient chemical reactions for sustainability. Ideal for students preparing for exams.
Explore the fundamentals of isotopes, including definitions, calculations of relative atomic mass, and the relationship between protons, neutrons, and mass number. This summary covers key concepts such as relative abundance and atomic structure, essential for mastering Module 2 in Chemistry.
Explore the fundamentals of atomic structure, relative atomic mass, and isotopes in this detailed summary. Understand concepts like average atomic mass, ionization, and the role of mass spectrometry in determining percentage abundance. Ideal for A Level Chemistry students preparing for exams.
C1 Chemistry (Atoms etc)
Explore the principles and methods of mass spectrometry, including electron impact and electrospray ionisation. Understand how molecular ions are formed, the significance of mass spectra, and how to calculate relative atomic mass. Ideal for AQA A-level chemistry students.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user