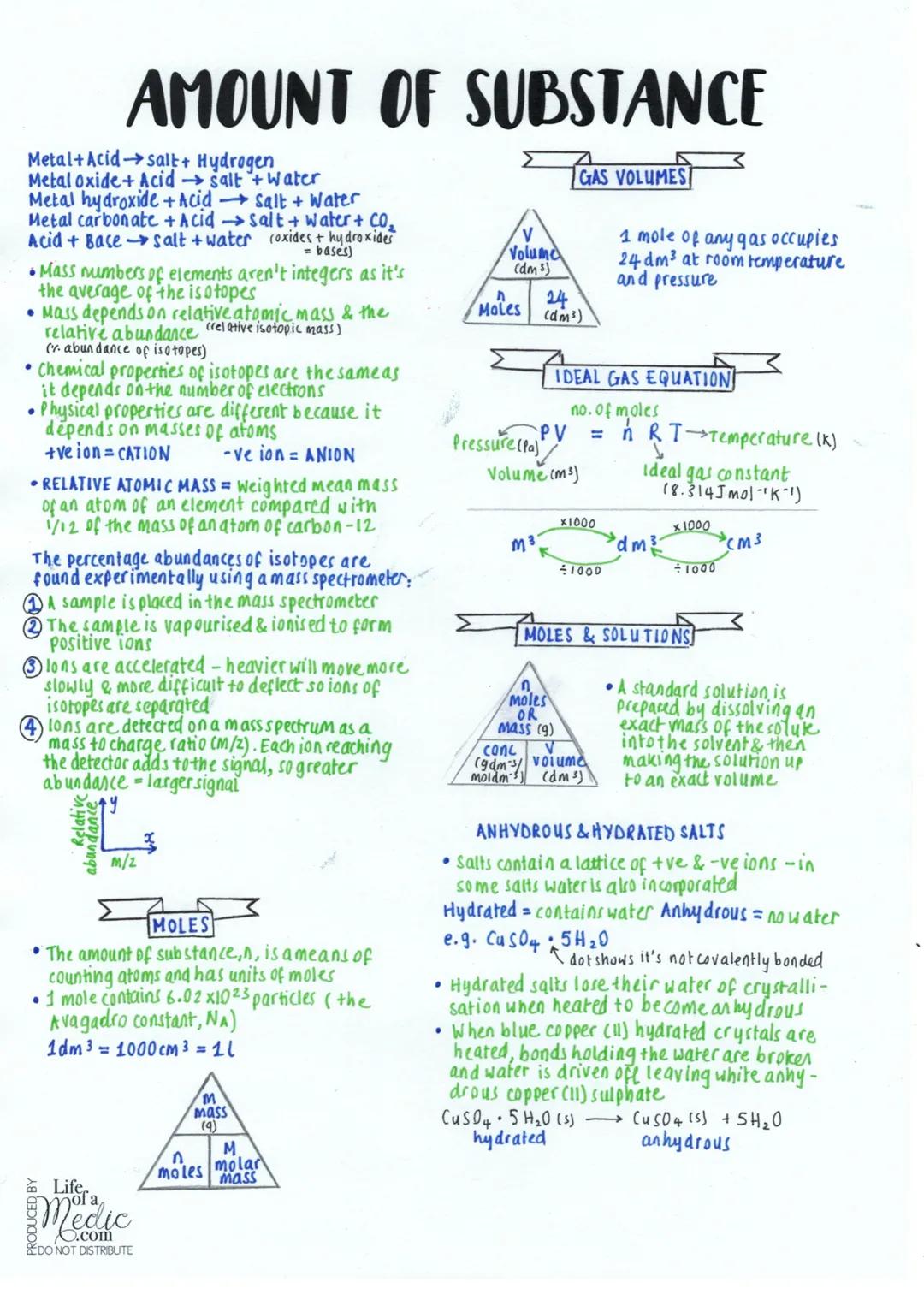
Moles and Gas Calculations
The mole is chemistry's way of counting ridiculously large numbers of atoms - one mole contains 6.02×10²³ particles (Avogadro's constant). Think of it like buying eggs by the dozen, but for atoms!
Gas volume calculations are surprisingly straightforward: one mole of any gas takes up 24 dm³ at room temperature and pressure. For more complex situations, you'll use the ideal gas equation: PV = nRT, where each letter represents pressure, volume, moles, gas constant, and temperature respectively.
Solutions and concentration calculations link mass, volume, and molarity. You can find moles by dividing mass by molar mass, or by multiplying concentration by volume. Standard solutions are made by dissolving an exact mass and diluting to an exact volume.
Hydrated salts contain water molecules trapped in their crystal structure (like CuSO₄·5H₂O). When heated, they lose this water and become anhydrous, often changing colour - blue copper sulfate turns white when dehydrated.
Remember: Always heat to constant mass when removing water to ensure you've driven off every last bit!