Proteins are absolutely everywhere in your body - from your... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
63
•
9 Feb 2026
•
Gabriela
@gabriela.my.school.journey16
Proteins are absolutely everywhere in your body - from your... Show more



Think of proteins as biological LEGO sets - they're massive, complex structures built from chains of smaller pieces called amino acids. These aren't just random molecules floating about; they're folded into incredibly specific 3D shapes that determine exactly what job they do in your body.
Proteins are basically the workhorses of life. They build your structural components like muscles, skin, and hair. Every single enzyme in your body is a protein, speeding up the chemical reactions that keep you alive. Many hormones and antibodies are proteins too, handling communication and immune defence.
The key thing to remember is that a functional protein contains one or more polypeptide chains - think of these as long strings of amino acids all linked together and then folded up like origami into the perfect shape for their job.
Quick Tip: If you can remember that "structure determines function," you'll understand why the precise folding of proteins is so crucial!
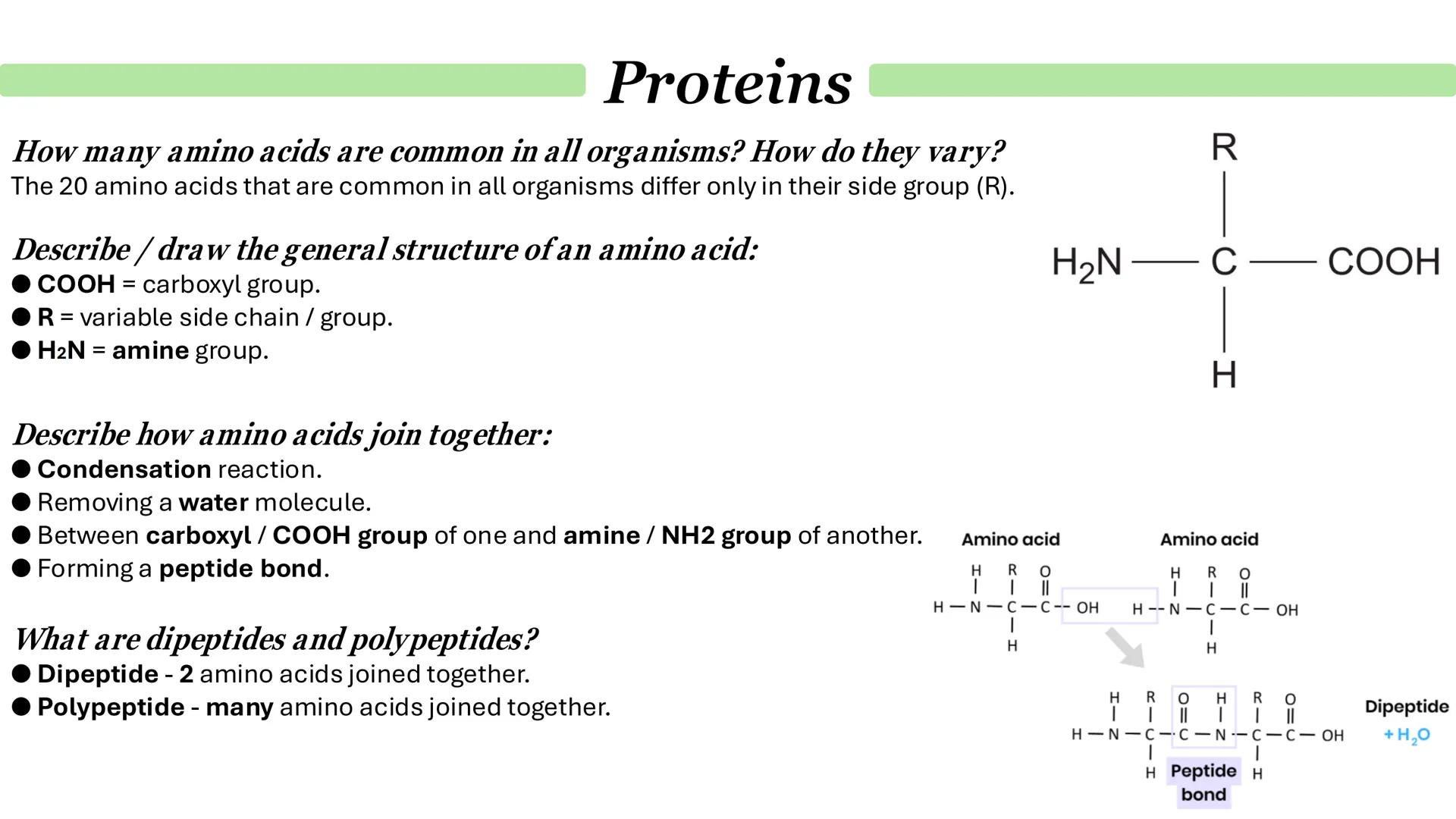
Here's something brilliant about biology - all life on Earth uses the same 20 amino acids as building blocks. They all have the same basic structure but differ only in their side group (R group), which is what gives each amino acid its unique properties.
Every amino acid has three key parts: an amine group (H2N), a carboxyl group (COOH), and that variable R group. When amino acids join together, they undergo a condensation reaction - basically, they kick out a water molecule and form a peptide bond between the carboxyl group of one and the amine group of another.
Start small and build up: two amino acids make a dipeptide, but keep adding more and you get a polypeptide. It's like building a chain - each new amino acid extends the sequence further.
Memory Trick: Remember "condensation = water out" - you're literally removing H2O to join amino acids together!
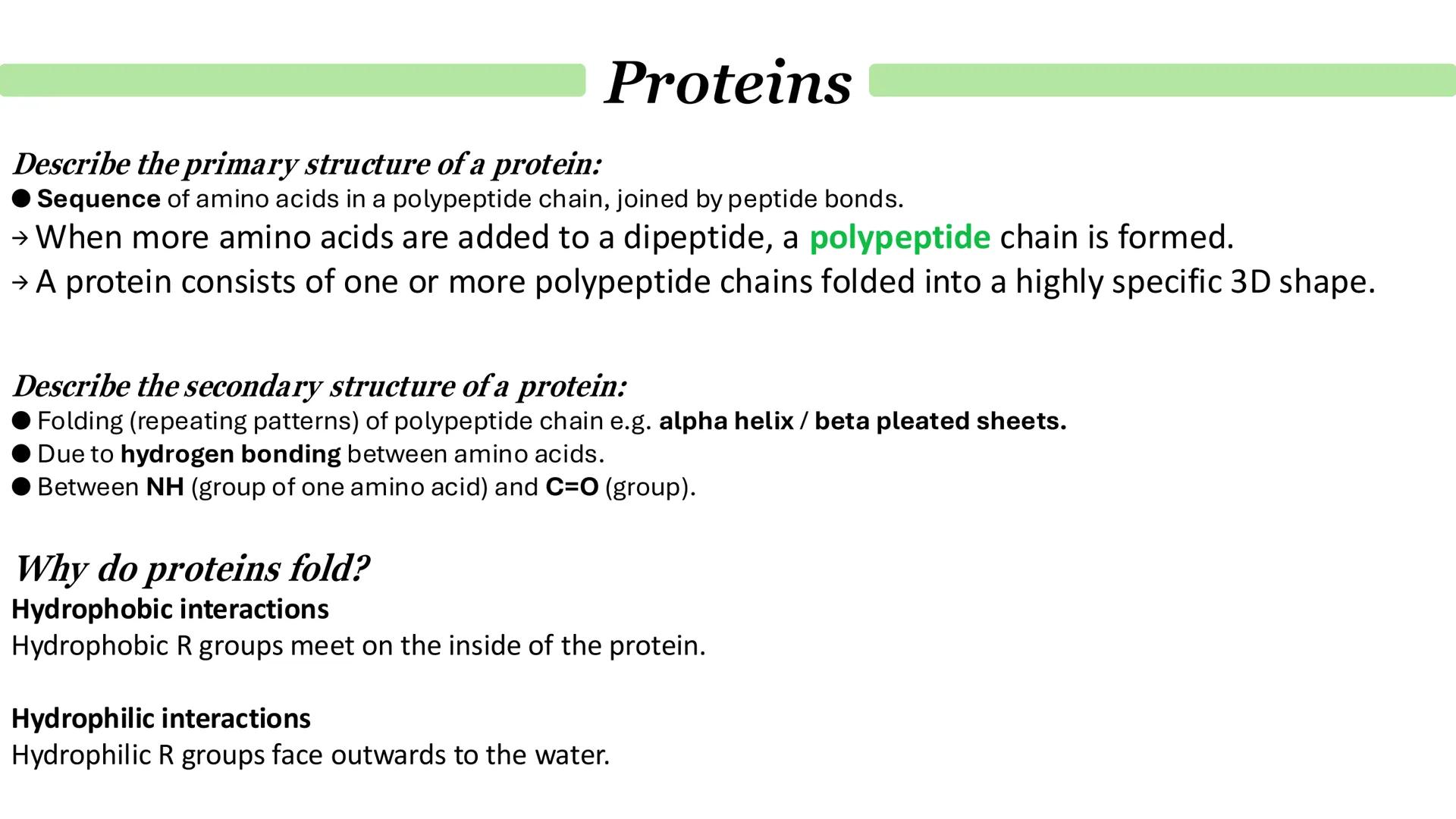
The primary structure is dead simple - it's just the sequence of amino acids in your polypeptide chain, held together by peptide bonds. Think of it as the linear "recipe" that determines everything else about the protein.
Secondary structure is where things get interesting. The polypeptide chain starts folding into repeating patterns like alpha helices (spiral staircases) or beta pleated sheets . This folding happens because of hydrogen bonding between the backbone atoms - specifically between NH groups and C=O groups.
But why do proteins bother folding at all? It's all about chemistry! Hydrophobic R groups try to hide on the inside of the protein, while hydrophilic R groups face outwards towards the watery environment. It's like people at a party - similar personalities cluster together.
Exam Focus: Remember that secondary structure is about backbone interactions, not side chain interactions - that comes later!
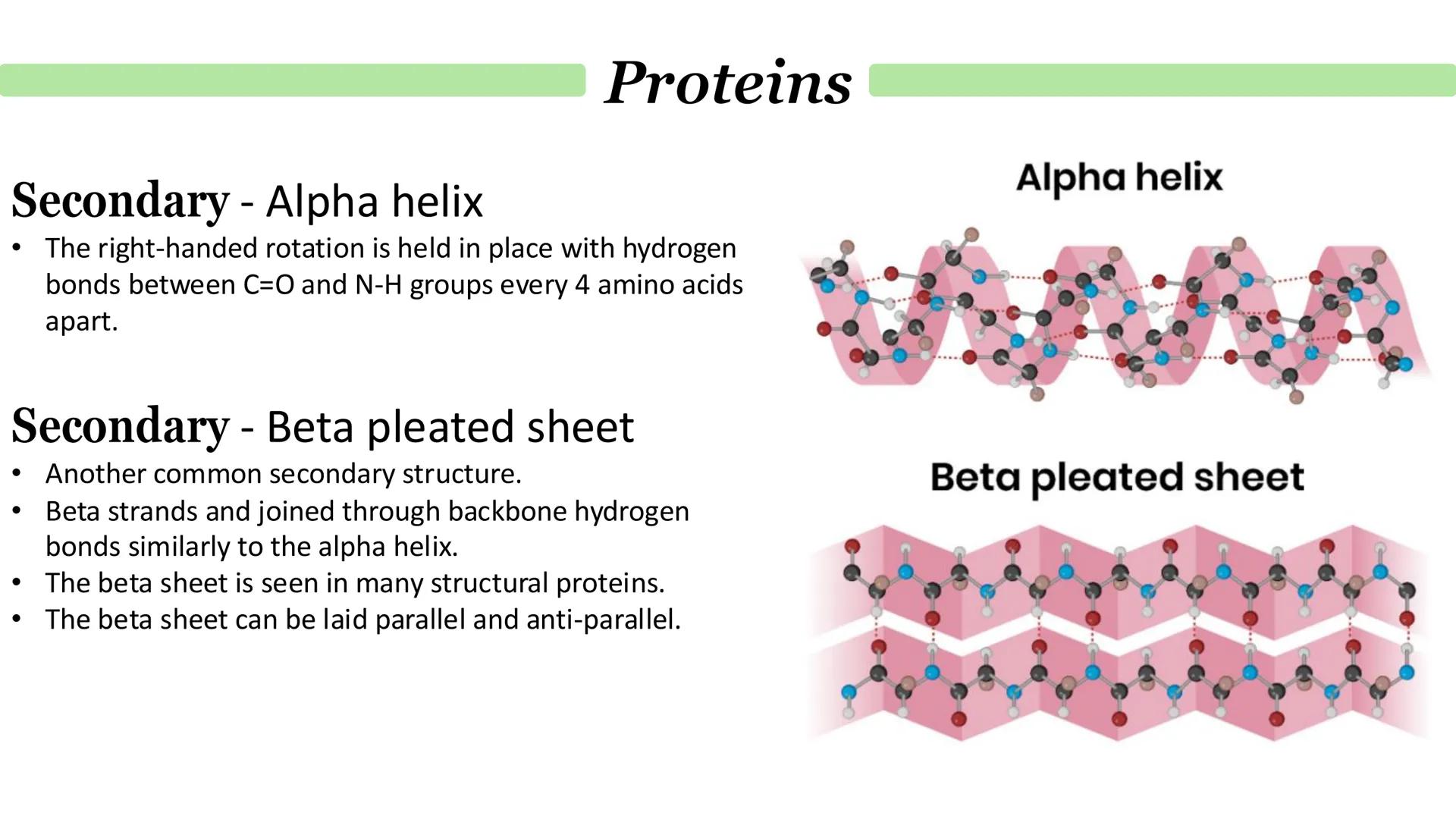
The alpha helix is like a right-handed spiral staircase held together by hydrogen bonds between amino acids that are exactly 4 positions apart. It's one of the most common and stable secondary structures you'll encounter.
Beta pleated sheets are completely different - imagine an accordion or folded paper. Here, different parts of the polypeptide chain (or even separate chains) lie alongside each other, connected by hydrogen bonds between their backbones.
Beta sheets can be arranged parallel (chains running in the same direction) or anti-parallel (chains running in opposite directions). You'll often find beta sheets in structural proteins that need to be strong and flexible.
Visual Tip: Alpha helices look like corkscrews, beta sheets look like corrugated cardboard - both held together by hydrogen bonds!
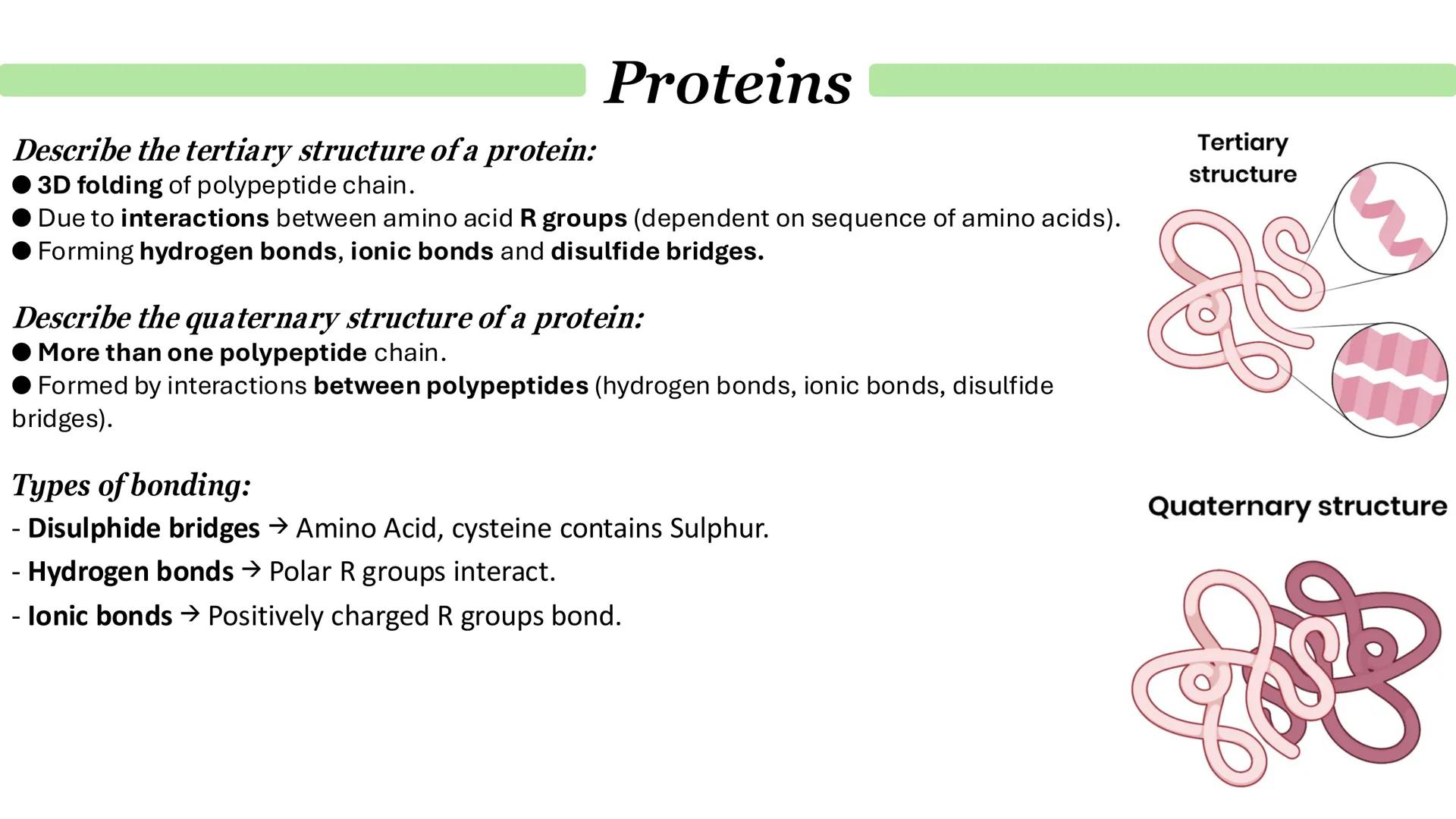
Tertiary structure is where your polypeptide chain folds into its final 3D shape. Unlike secondary structure, this folding involves interactions between the R groups of amino acids that might be far apart in the sequence but end up close together when folded.
Three main types of bonds create tertiary structure: disulfide bridges (between cysteine amino acids containing sulfur), hydrogen bonds (between polar R groups), and ionic bonds (between oppositely charged R groups).
Quaternary structure only exists in proteins made from multiple polypeptide chains. Think of haemoglobin - it's got four separate polypeptide subunits all working together. Each subunit has its own primary, secondary, and tertiary structure, but they're held together by the same types of bonds.
Key Point: Not all proteins have quaternary structure - only those with multiple polypeptide chains qualify!

Some proteins are seriously complex, with multiple polypeptide subunits packed together like puzzle pieces. Each subunit is a complete protein in its own right, with all four levels of structure, but they work together as a team.
Haemoglobin and insulin are classic examples you need to know. These subunits stick together through hydrogen bonds and Van Der Waals forces - relatively weak interactions that allow some flexibility while maintaining the overall structure.
Many complex proteins also have prosthetic groups - non-protein components that are essential for function. Haemoglobin's haem group contains iron and is what actually binds oxygen. Without it, the protein would be useless for oxygen transport.
Real-World Connection: This is why carbon monoxide poisoning is deadly - CO binds to haemoglobin's iron more strongly than oxygen does!
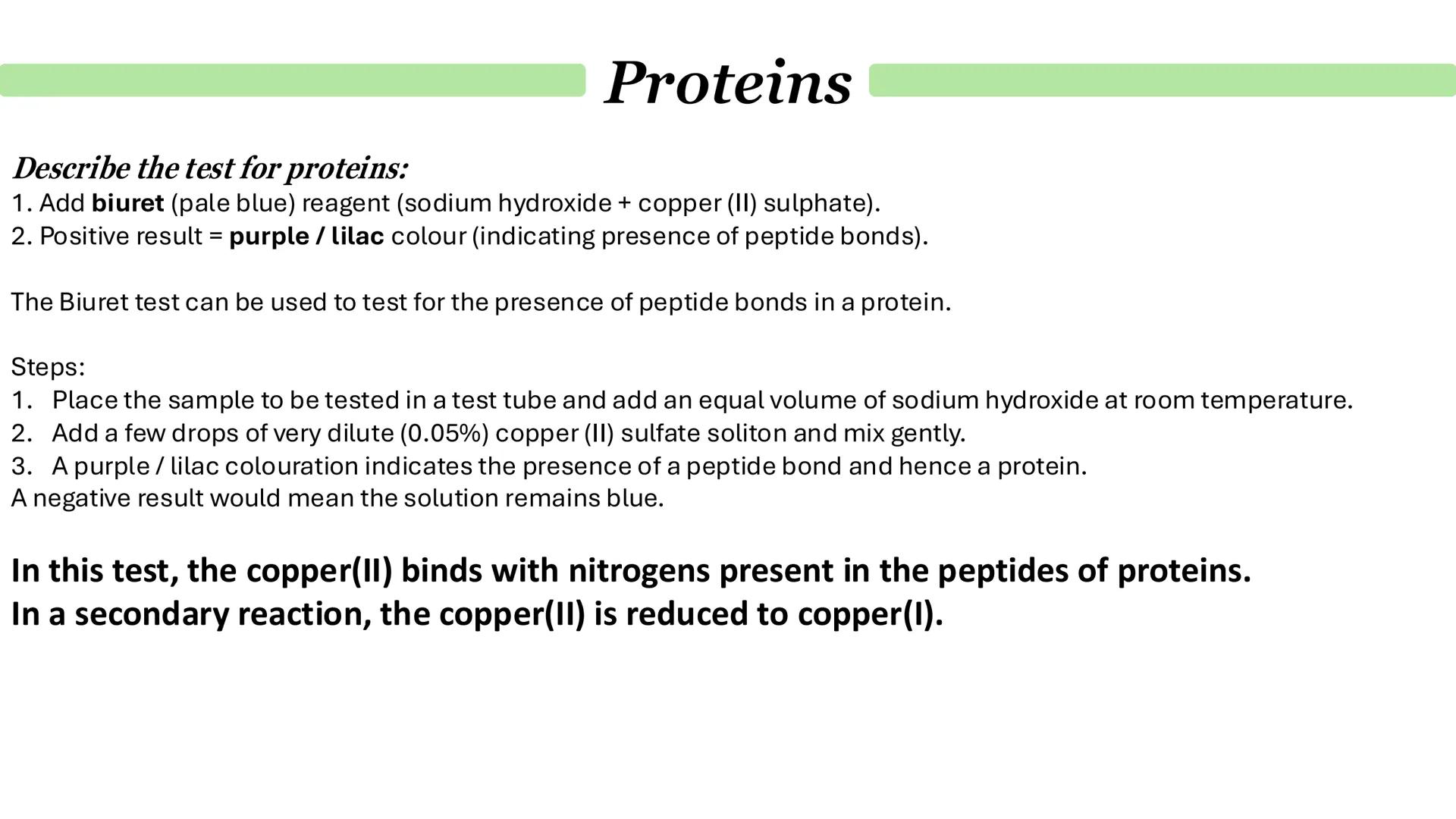
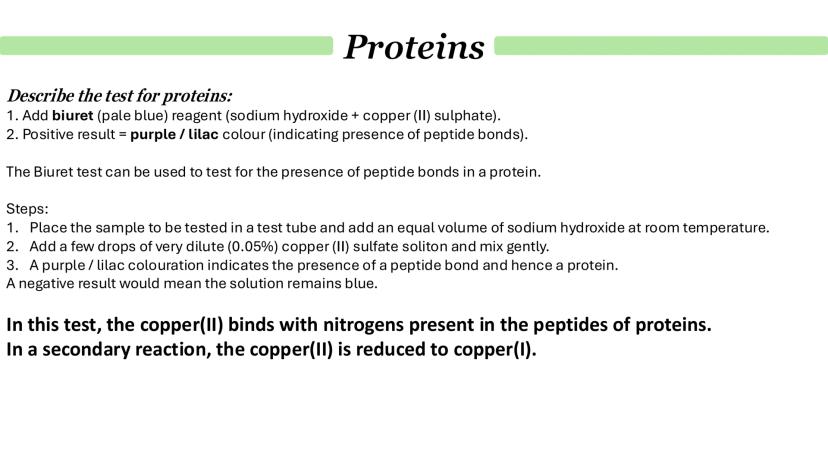
The biuret test is your go-to method for detecting proteins, and it's actually testing for peptide bonds rather than the proteins themselves. Start with biuret reagent (sodium hydroxide plus copper sulfate), which is pale blue.
Here's the method: add equal volumes of your sample and sodium hydroxide, then add a few drops of dilute copper sulfate solution. Mix gently and look for a colour change. A positive result gives you a purple or lilac colour, while a negative result stays blue.
The science behind it is quite neat - the copper ions bind to nitrogen atoms in the peptide bonds. In a secondary reaction, copper(II) gets reduced to copper(I), which causes the characteristic colour change.
Lab Tip: Always add the copper sulfate drop by drop - too much can give confusing results and waste your sample!
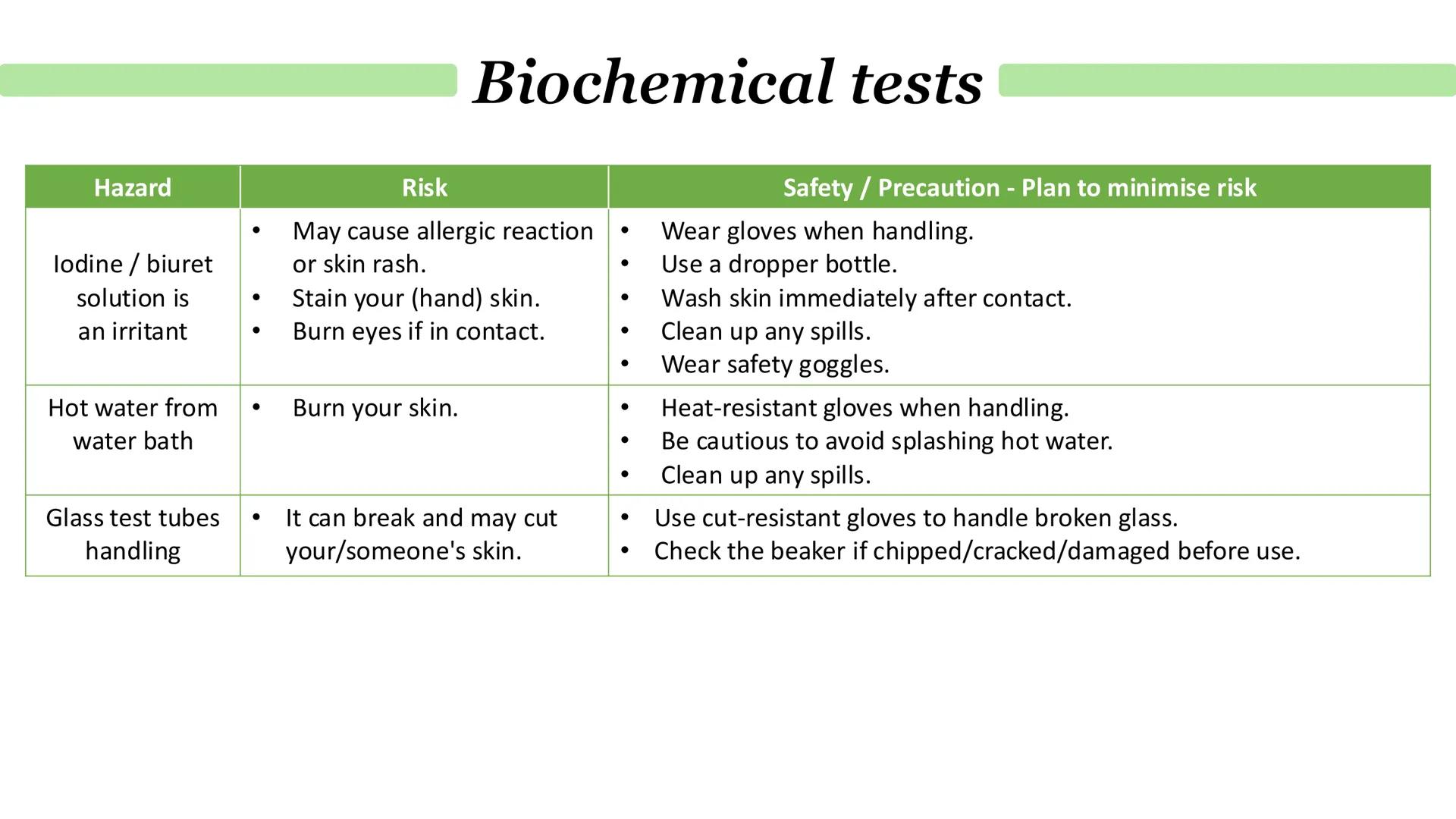
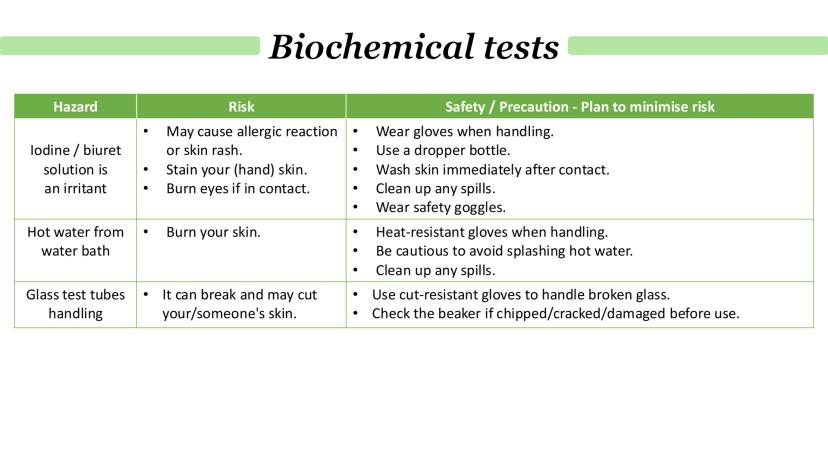
When you're doing biochemical tests, safety isn't just important - it's essential. Iodine and biuret solutions are irritants that can cause allergic reactions, skin rashes, and serious eye damage if you're not careful.
Hot water baths pose obvious burn risks to your skin, and there's always the danger of splashing. Glass test tubes can break and cut you, especially when heated or handled roughly.
Your safety strategy should include wearing gloves when handling chemicals, using dropper bottles to control amounts, and washing skin immediately after any contact. Always wear safety goggles, use heat-resistant gloves with hot equipment, and check glassware for damage before use.
Safety First: Clean up spills immediately - they're slip hazards and can cause chemical exposure for the next person!
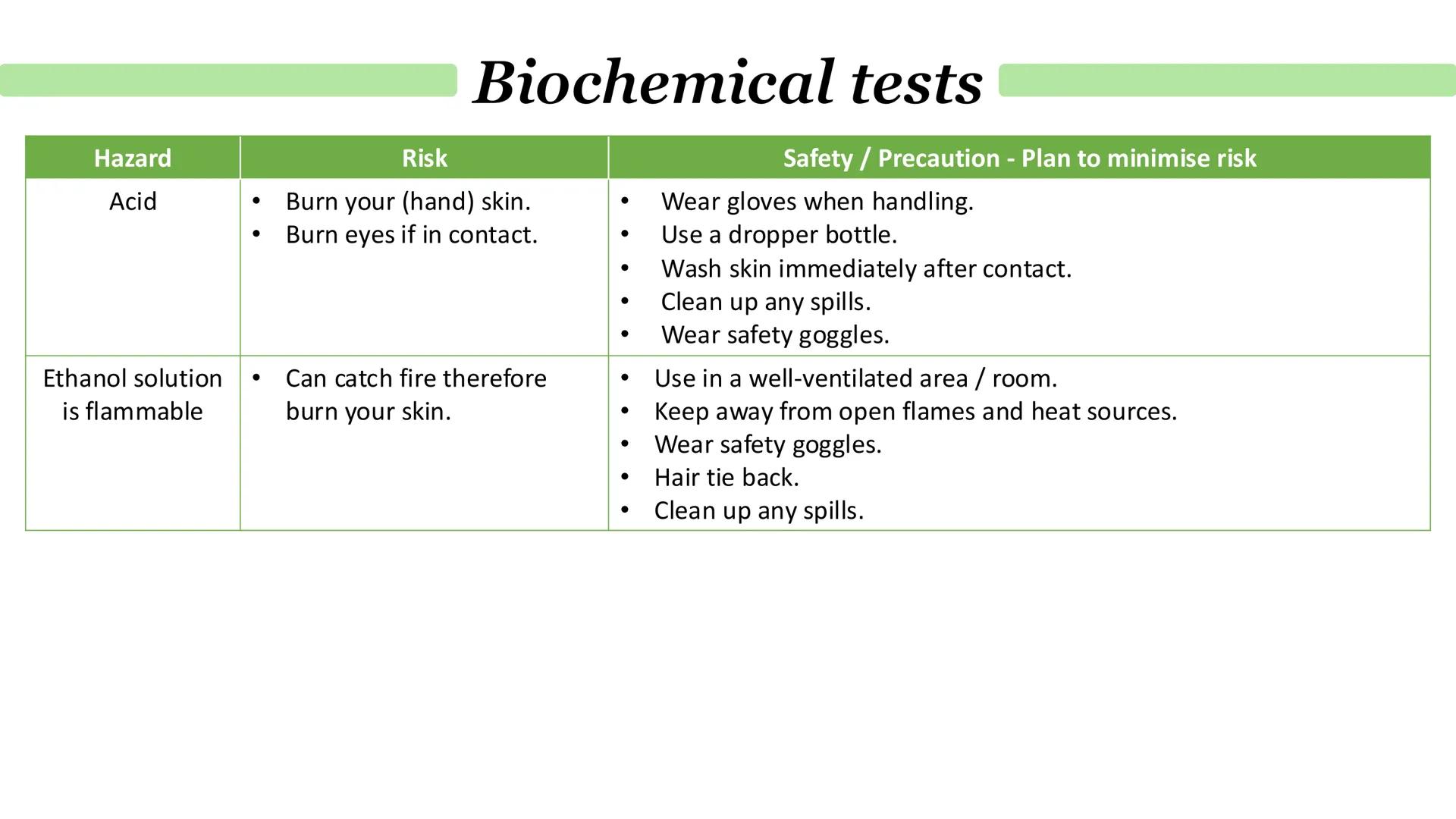
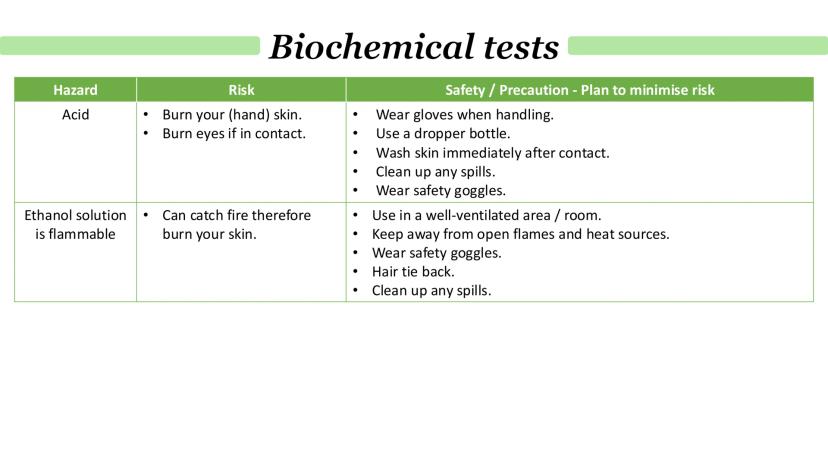
Acids are serious business in the lab - they'll burn your skin and eyes, so treat them with respect. Always wear gloves and safety goggles, use dropper bottles for control, and wash off any contact immediately.
Ethanol solutions bring fire risk into the equation. They're highly flammable and can catch fire easily, potentially causing serious burns. The key is keeping them away from any heat sources or open flames.
Good lab practice means working in well-ventilated areas, tying back long hair, wearing appropriate safety gear, and cleaning up spills straight away. Remember that accidents happen when people get complacent, so stay alert.
Emergency Protocol: Know where the eyewash stations and fire blankets are before you start any practical work!
Globular proteins are the celebrities of the protein world - compact, roughly spherical, and crucially, soluble in water. They achieve this spherical shape through clever folding that puts hydrophobic R groups on the inside and hydrophilic R groups on the outside.
This inside-out arrangement is brilliant because water molecules can surround and interact with the polar groups on the surface. That's why globular proteins can be easily transported around your body and participate in metabolic reactions - they actually dissolve in your blood and cellular fluids.
The specific shapes of globular proteins are what make them so useful. Enzymes can catalyse particular reactions because their shape fits perfectly with their substrate. Antibodies can recognise specific antigens for the same reason.
Many globular proteins are conjugated proteins with prosthetic groups attached - haemoglobin with its haem group is the perfect example.
Function Focus: Remember that globular proteins are the "doers" - enzymes, hormones, antibodies - while fibrous proteins are the "builders" like collagen and keratin!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Gabriela
@gabriela.my.school.journey16
Proteins are absolutely everywhere in your body - from your muscles and hair to the enzymes that keep you alive. Understanding how these complex molecules are built from simple amino acid building blocks will help you grasp one of biology's... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of proteins as biological LEGO sets - they're massive, complex structures built from chains of smaller pieces called amino acids. These aren't just random molecules floating about; they're folded into incredibly specific 3D shapes that determine exactly what job they do in your body.
Proteins are basically the workhorses of life. They build your structural components like muscles, skin, and hair. Every single enzyme in your body is a protein, speeding up the chemical reactions that keep you alive. Many hormones and antibodies are proteins too, handling communication and immune defence.
The key thing to remember is that a functional protein contains one or more polypeptide chains - think of these as long strings of amino acids all linked together and then folded up like origami into the perfect shape for their job.
Quick Tip: If you can remember that "structure determines function," you'll understand why the precise folding of proteins is so crucial!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
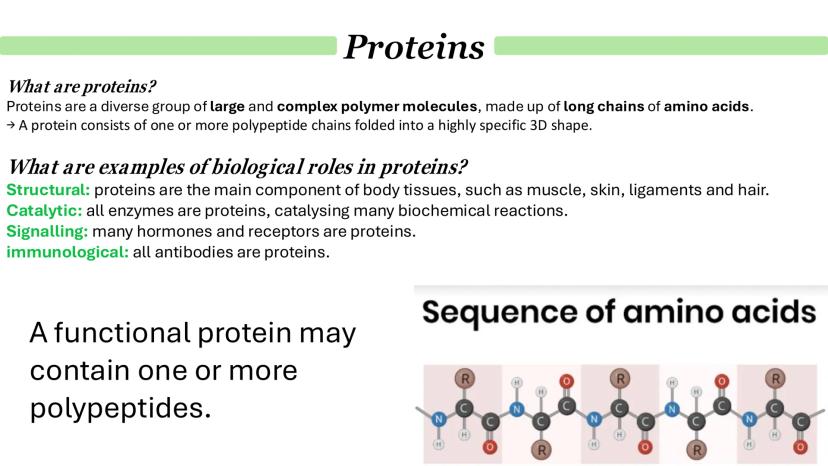
Here's something brilliant about biology - all life on Earth uses the same 20 amino acids as building blocks. They all have the same basic structure but differ only in their side group (R group), which is what gives each amino acid its unique properties.
Every amino acid has three key parts: an amine group (H2N), a carboxyl group (COOH), and that variable R group. When amino acids join together, they undergo a condensation reaction - basically, they kick out a water molecule and form a peptide bond between the carboxyl group of one and the amine group of another.
Start small and build up: two amino acids make a dipeptide, but keep adding more and you get a polypeptide. It's like building a chain - each new amino acid extends the sequence further.
Memory Trick: Remember "condensation = water out" - you're literally removing H2O to join amino acids together!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The primary structure is dead simple - it's just the sequence of amino acids in your polypeptide chain, held together by peptide bonds. Think of it as the linear "recipe" that determines everything else about the protein.
Secondary structure is where things get interesting. The polypeptide chain starts folding into repeating patterns like alpha helices (spiral staircases) or beta pleated sheets . This folding happens because of hydrogen bonding between the backbone atoms - specifically between NH groups and C=O groups.
But why do proteins bother folding at all? It's all about chemistry! Hydrophobic R groups try to hide on the inside of the protein, while hydrophilic R groups face outwards towards the watery environment. It's like people at a party - similar personalities cluster together.
Exam Focus: Remember that secondary structure is about backbone interactions, not side chain interactions - that comes later!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
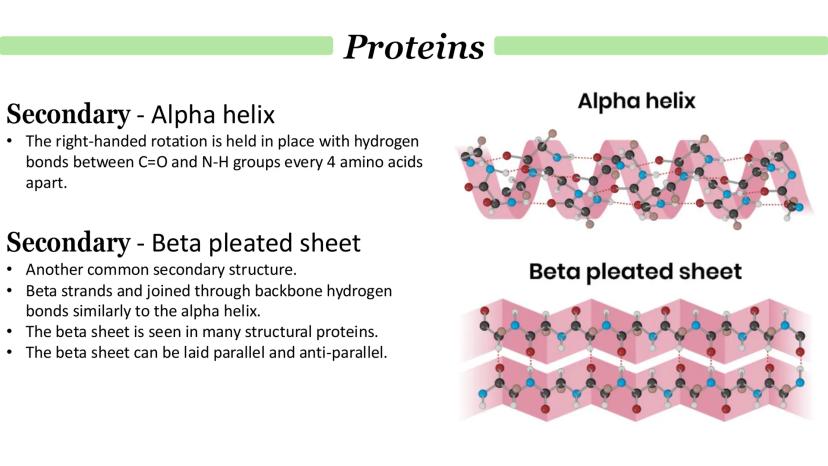
The alpha helix is like a right-handed spiral staircase held together by hydrogen bonds between amino acids that are exactly 4 positions apart. It's one of the most common and stable secondary structures you'll encounter.
Beta pleated sheets are completely different - imagine an accordion or folded paper. Here, different parts of the polypeptide chain (or even separate chains) lie alongside each other, connected by hydrogen bonds between their backbones.
Beta sheets can be arranged parallel (chains running in the same direction) or anti-parallel (chains running in opposite directions). You'll often find beta sheets in structural proteins that need to be strong and flexible.
Visual Tip: Alpha helices look like corkscrews, beta sheets look like corrugated cardboard - both held together by hydrogen bonds!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
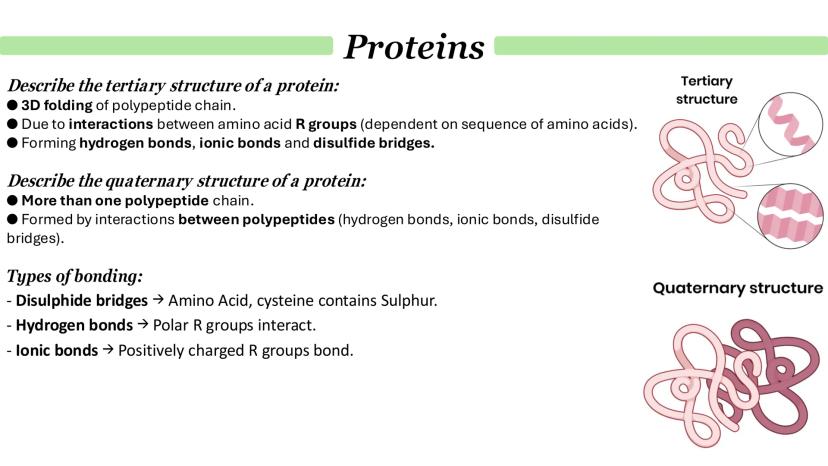
Tertiary structure is where your polypeptide chain folds into its final 3D shape. Unlike secondary structure, this folding involves interactions between the R groups of amino acids that might be far apart in the sequence but end up close together when folded.
Three main types of bonds create tertiary structure: disulfide bridges (between cysteine amino acids containing sulfur), hydrogen bonds (between polar R groups), and ionic bonds (between oppositely charged R groups).
Quaternary structure only exists in proteins made from multiple polypeptide chains. Think of haemoglobin - it's got four separate polypeptide subunits all working together. Each subunit has its own primary, secondary, and tertiary structure, but they're held together by the same types of bonds.
Key Point: Not all proteins have quaternary structure - only those with multiple polypeptide chains qualify!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Some proteins are seriously complex, with multiple polypeptide subunits packed together like puzzle pieces. Each subunit is a complete protein in its own right, with all four levels of structure, but they work together as a team.
Haemoglobin and insulin are classic examples you need to know. These subunits stick together through hydrogen bonds and Van Der Waals forces - relatively weak interactions that allow some flexibility while maintaining the overall structure.
Many complex proteins also have prosthetic groups - non-protein components that are essential for function. Haemoglobin's haem group contains iron and is what actually binds oxygen. Without it, the protein would be useless for oxygen transport.
Real-World Connection: This is why carbon monoxide poisoning is deadly - CO binds to haemoglobin's iron more strongly than oxygen does!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The biuret test is your go-to method for detecting proteins, and it's actually testing for peptide bonds rather than the proteins themselves. Start with biuret reagent (sodium hydroxide plus copper sulfate), which is pale blue.
Here's the method: add equal volumes of your sample and sodium hydroxide, then add a few drops of dilute copper sulfate solution. Mix gently and look for a colour change. A positive result gives you a purple or lilac colour, while a negative result stays blue.
The science behind it is quite neat - the copper ions bind to nitrogen atoms in the peptide bonds. In a secondary reaction, copper(II) gets reduced to copper(I), which causes the characteristic colour change.
Lab Tip: Always add the copper sulfate drop by drop - too much can give confusing results and waste your sample!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When you're doing biochemical tests, safety isn't just important - it's essential. Iodine and biuret solutions are irritants that can cause allergic reactions, skin rashes, and serious eye damage if you're not careful.
Hot water baths pose obvious burn risks to your skin, and there's always the danger of splashing. Glass test tubes can break and cut you, especially when heated or handled roughly.
Your safety strategy should include wearing gloves when handling chemicals, using dropper bottles to control amounts, and washing skin immediately after any contact. Always wear safety goggles, use heat-resistant gloves with hot equipment, and check glassware for damage before use.
Safety First: Clean up spills immediately - they're slip hazards and can cause chemical exposure for the next person!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Acids are serious business in the lab - they'll burn your skin and eyes, so treat them with respect. Always wear gloves and safety goggles, use dropper bottles for control, and wash off any contact immediately.
Ethanol solutions bring fire risk into the equation. They're highly flammable and can catch fire easily, potentially causing serious burns. The key is keeping them away from any heat sources or open flames.
Good lab practice means working in well-ventilated areas, tying back long hair, wearing appropriate safety gear, and cleaning up spills straight away. Remember that accidents happen when people get complacent, so stay alert.
Emergency Protocol: Know where the eyewash stations and fire blankets are before you start any practical work!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Globular proteins are the celebrities of the protein world - compact, roughly spherical, and crucially, soluble in water. They achieve this spherical shape through clever folding that puts hydrophobic R groups on the inside and hydrophilic R groups on the outside.
This inside-out arrangement is brilliant because water molecules can surround and interact with the polar groups on the surface. That's why globular proteins can be easily transported around your body and participate in metabolic reactions - they actually dissolve in your blood and cellular fluids.
The specific shapes of globular proteins are what make them so useful. Enzymes can catalyse particular reactions because their shape fits perfectly with their substrate. Antibodies can recognise specific antigens for the same reason.
Many globular proteins are conjugated proteins with prosthetic groups attached - haemoglobin with its haem group is the perfect example.
Function Focus: Remember that globular proteins are the "doers" - enzymes, hormones, antibodies - while fibrous proteins are the "builders" like collagen and keratin!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
1
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the four levels of protein structure: primary, secondary, tertiary, and quaternary. This detailed summary covers key concepts such as peptide bonds, hydrogen bonds, and the differences between globular and fibrous proteins. Ideal for A Level Biology students preparing for exams.
Explore the intricate world of proteins with this detailed summary covering their structures, functions, and the role of amino acids. Understand primary, secondary, tertiary, and quaternary structures, along with key concepts like peptide bonds, denaturation, and enzyme functions. Ideal for AQA A-level biology students.
Explore the essential concepts of biological molecules, including DNA replication, protein structure, and the roles of carbohydrates and lipids. This comprehensive summary covers key processes such as semiconservative replication, enzyme functions, and the significance of nucleotides in nucleic acids. Ideal for OCR A Level Biology students, this resource aligns with the 2016-onwards specification and provides a clear understanding of molecular biology fundamentals.
Explore the intricate structure of proteins, including amino acid composition, peptide bonds, and the four levels of protein structure: primary, secondary, tertiary, and quaternary. This summary is essential for OCR A Level Biology, covering key concepts such as protein functions, enzyme roles, and the significance of various bonds in maintaining protein shape.
Explore the intricate world of proteins, including their structure, functions, and the role of amino acids. This summary covers key concepts such as peptide bonds, denaturation, and the four levels of protein organization. Ideal for A-Level Biology students studying biological molecules.
Explore the key digestive enzymes: Amylase, Protease, and Lipase. This summary covers their functions, production sites, and optimal pH levels for activity. Ideal for students studying human biology and digestive processes.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user