Ever wonder what makes your DNA different from a piece... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
379
•
6 Feb 2026
•
M
@iamjungkook
Ever wonder what makes your DNA different from a piece... Show more







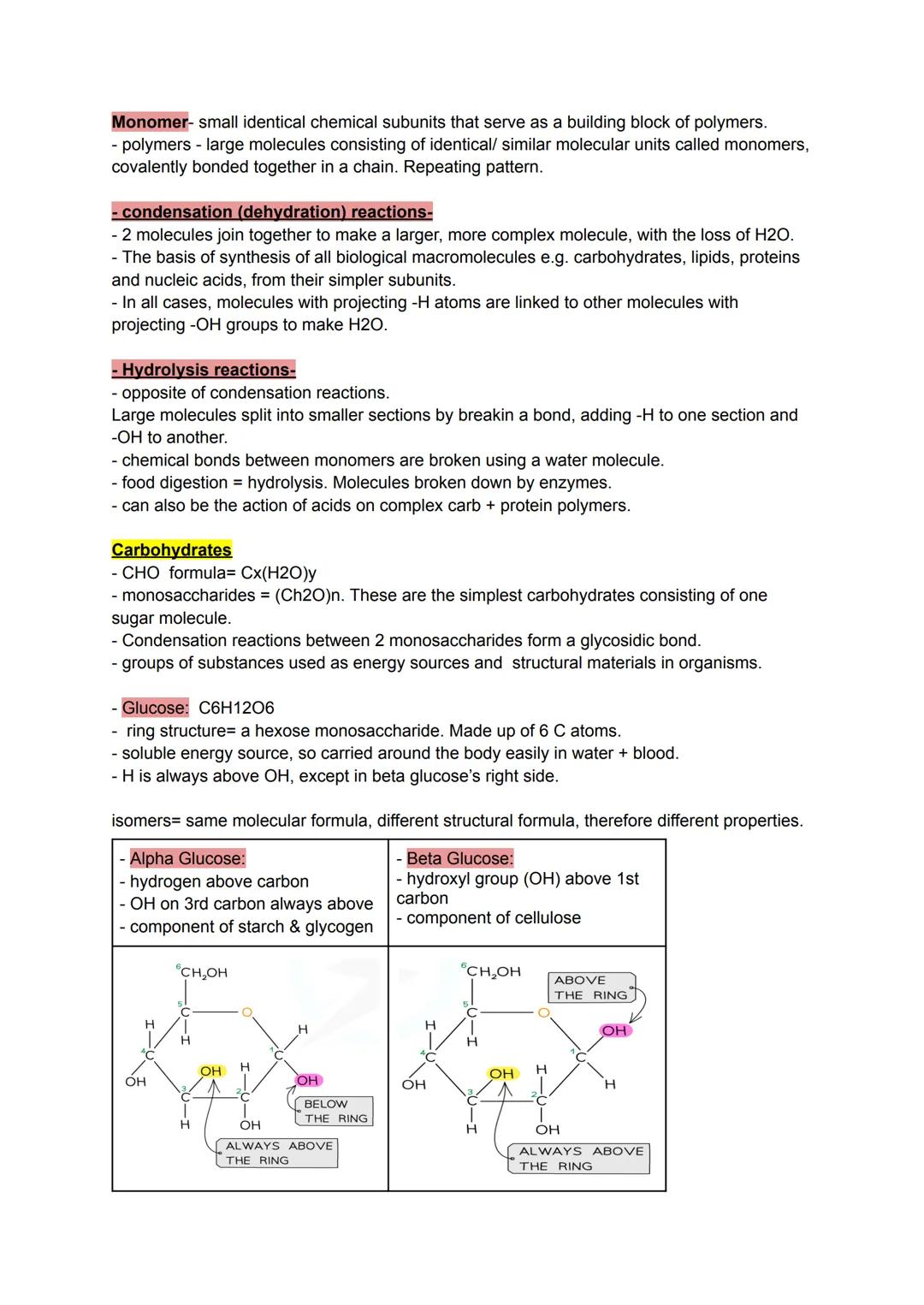
Think of biological molecules like construction sets - monomers are the individual bricks, whilst polymers are the finished structures built from identical pieces joined together. This simple concept explains how your body creates everything from energy stores to muscle fibres.
Two key reactions control this molecular construction site. Condensation reactions join monomers together by removing water molecules, like welding pieces with the loss of steam. Hydrolysis reactions do the opposite - they break apart large molecules by adding water back, which is exactly what happens when you digest food.
Carbohydrates follow the CHO formula and serve as your body's primary energy source. Monosaccharides like glucose are single sugar units that dissolve easily in blood for quick transport around your body.
Quick Tip: Remember that alpha glucose (used in starch) has hydrogen above the first carbon, whilst beta glucose (used in cellulose) has the OH group above - this tiny difference completely changes their properties!
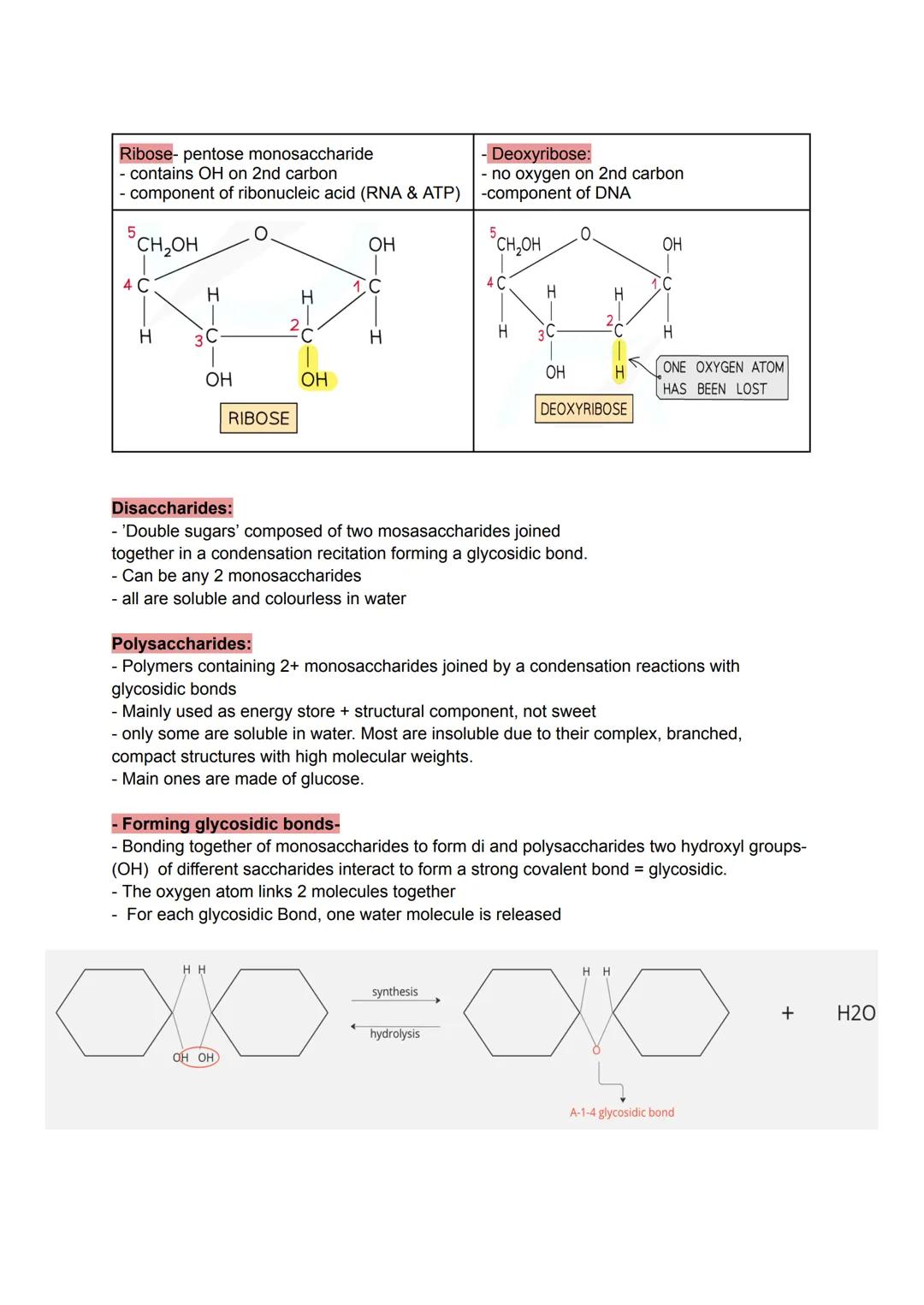
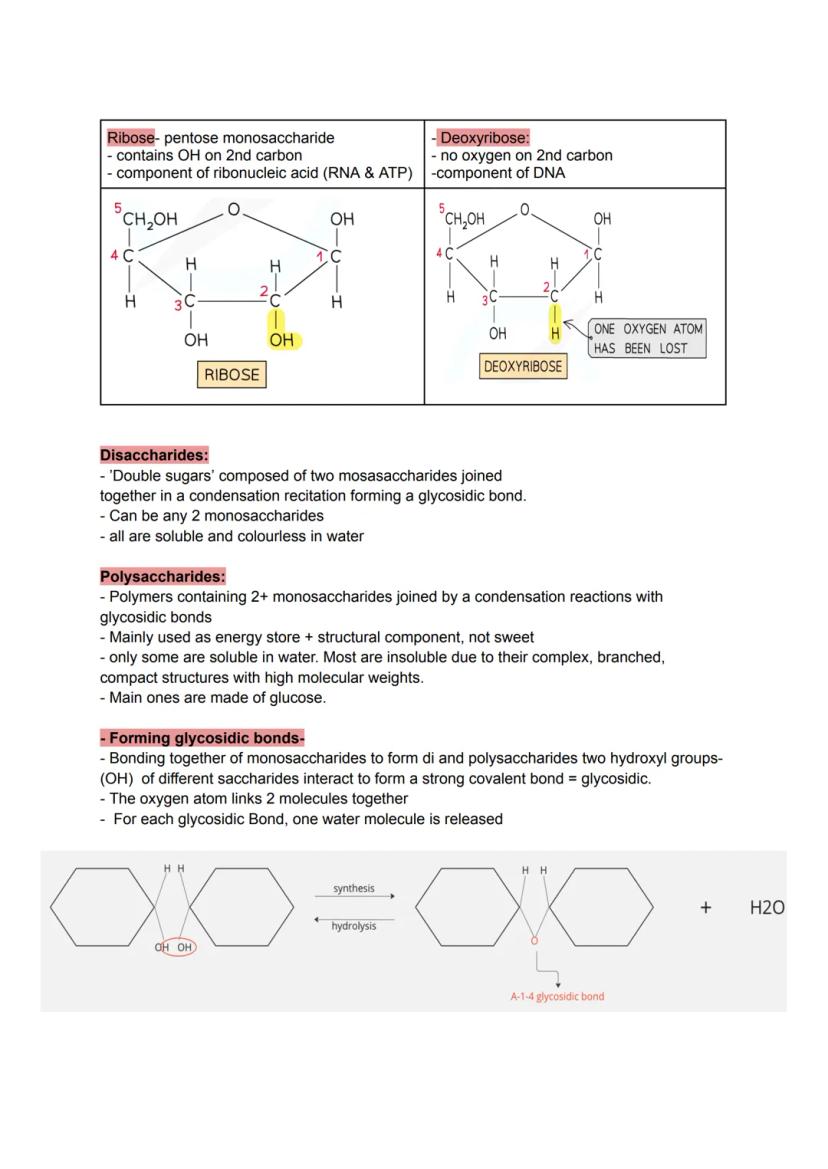
Ribose and deoxyribose might sound complicated, but they're just five-carbon sugars with a crucial difference. Ribose (in RNA) keeps all its OH groups, whilst deoxyribose (in DNA) loses one oxygen atom - making DNA more stable for long-term information storage.
When two monosaccharides join through condensation reactions, they form glycosidic bonds and create disaccharides like table sugar. These double sugars remain soluble and sweet, perfect for quick energy hits.
Polysaccharides are the heavy-duty storage units - long chains of sugars linked by glycosidic bonds. Most are insoluble due to their massive, branched structures, which makes them ideal for storing energy without affecting your cells' water balance.
Exam Focus: Each glycosidic bond formation releases one water molecule - count the bonds to work out how much water is produced in synthesis reactions!
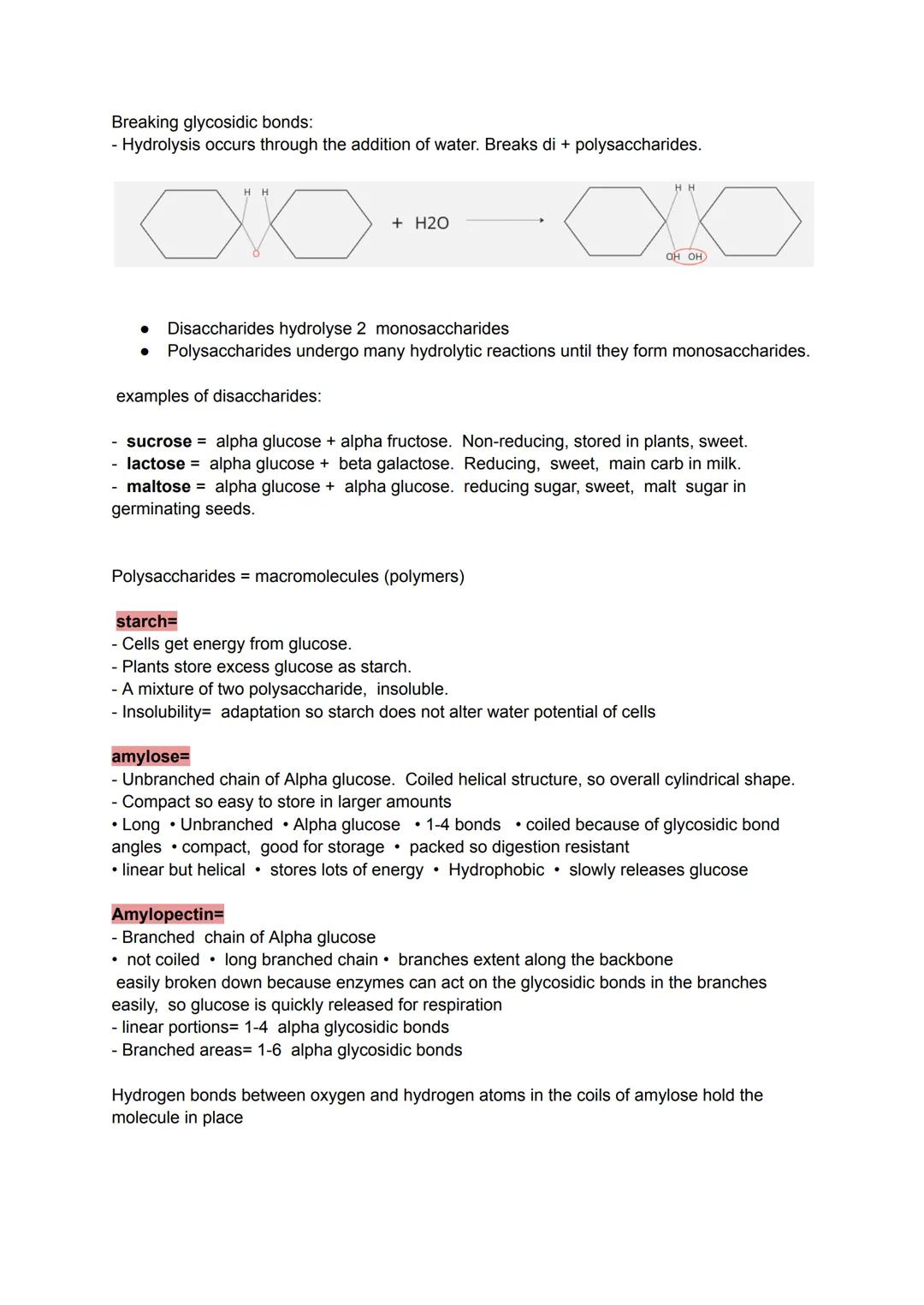
Plants face the same problem you do - they need to store excess energy for later use. Starch solves this perfectly by being completely insoluble, so it won't mess with the plant's water balance while storing massive amounts of glucose.
Amylose forms unbranched chains that coil into tight spirals, packing efficiently like a compressed spring. Its compact structure makes it brilliant for storage but slow to break down. Amylopectin takes a different approach with its branched design, allowing enzymes to attack multiple points simultaneously for faster glucose release.
The branching happens through different bond types - straight sections use 1-4 glycosidic bonds, whilst branch points use 1-6 bonds. This creates a tree-like structure that balances storage efficiency with accessibility.
Memory Trick: Amylose = "A-my-coiled" (coiled structure), Amylopectin = "A-my-branches" (branched structure)!

Glycogen is basically amylopectin on steroids - it has branches every 8-12 glucose units compared to starch's longer straight sections. This ultra-branched structure reflects animals' higher metabolic demands and need for rapid energy release during movement.
Cellulose breaks all the rules by using beta glucose, creating straight, unbranched chains where every other glucose molecule flips 180°. This alternating pattern allows adjacent molecules to form hydrogen bonds, creating rope-like microfibrils of incredible strength.
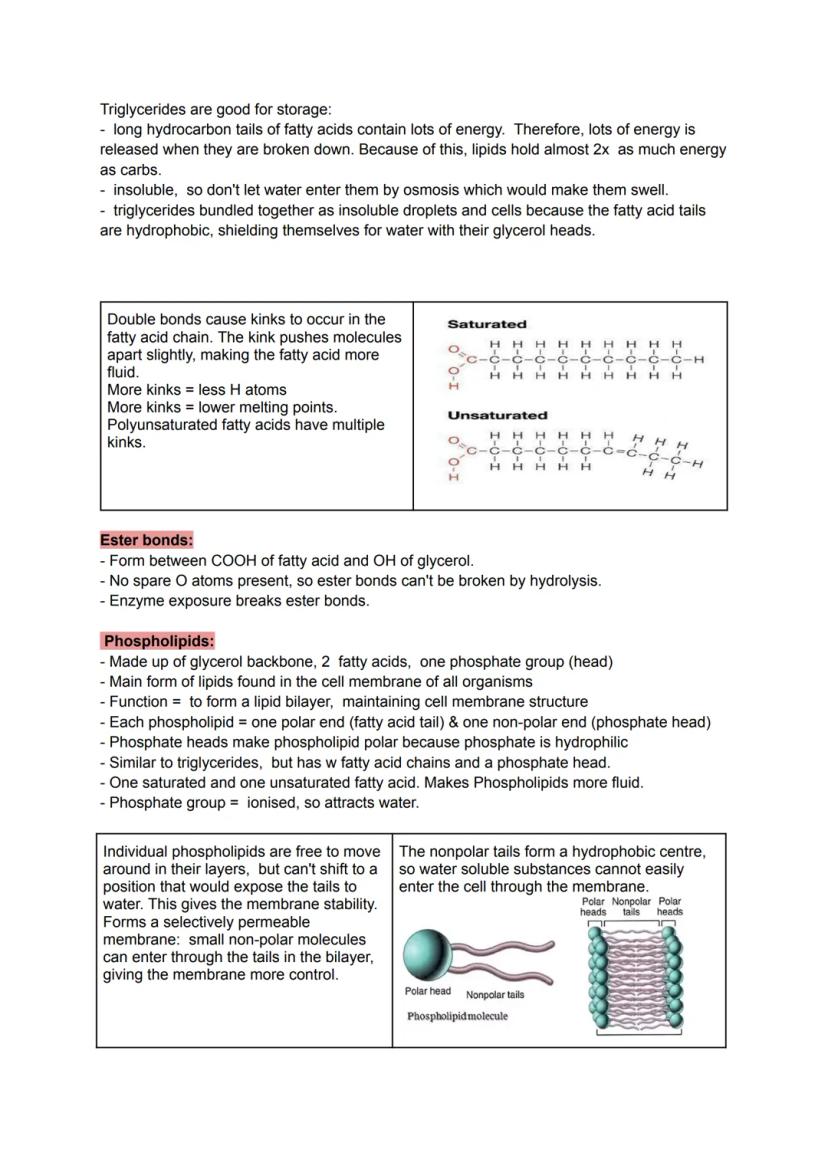
Triglycerides represent a completely different storage strategy. Made from glycerol plus three fatty acids joined by ester bonds, they pack almost twice the energy of carbohydrates. Their hydrophobic nature means they won't absorb water and swell up like a sponge.
Real-world Connection: Cellulose's strength-to-weight ratio rivals steel - that's why it's perfect for plant cell walls and why we use it to make paper!
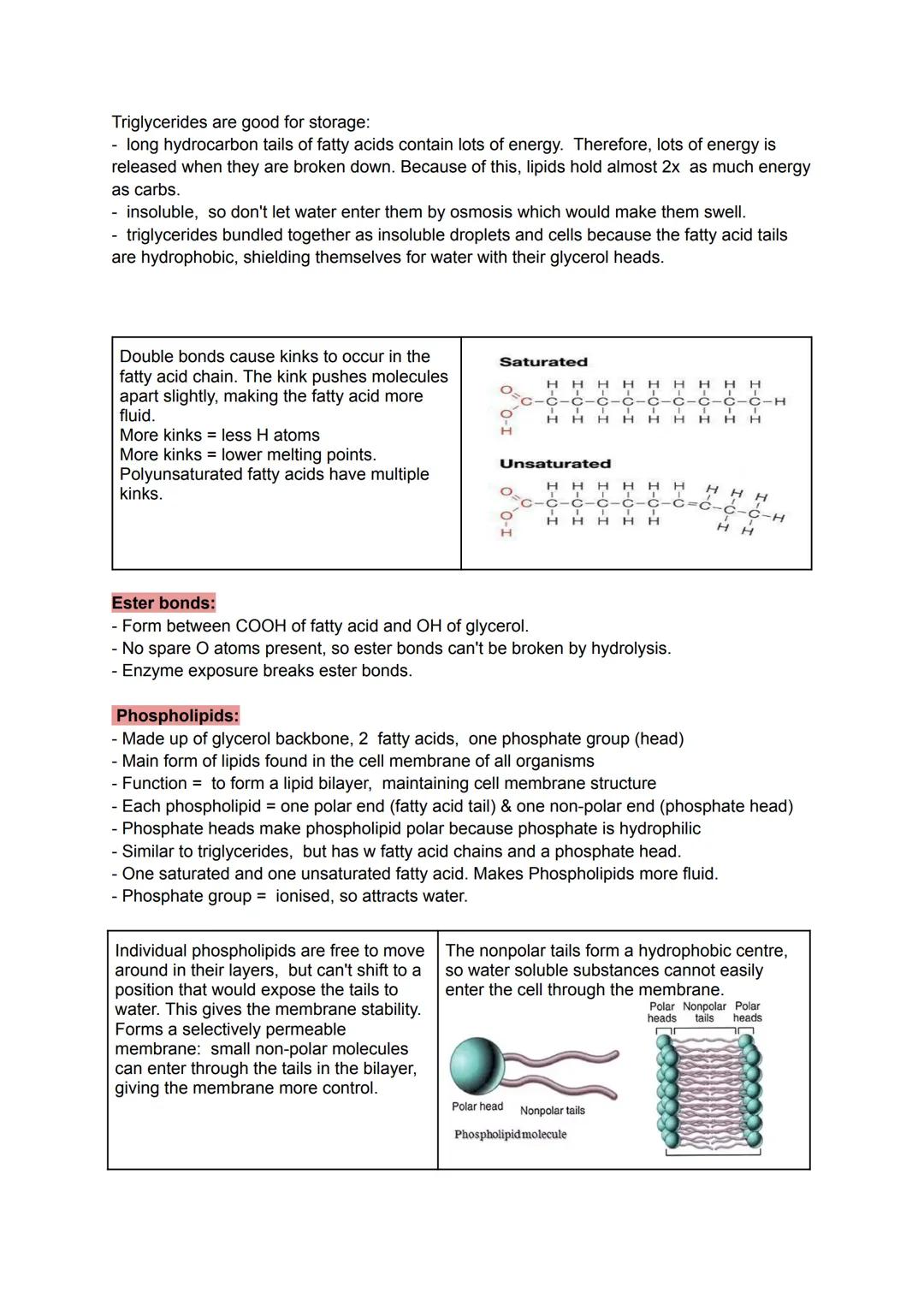
Saturated fatty acids pack together tightly like straight pencils in a box, whilst unsaturated fatty acids have kinks from double bonds that push molecules apart. More kinks mean lower melting points - think of butter (saturated) versus olive oil (unsaturated) at room temperature.
Phospholipids are the molecular architects of cell membranes. With hydrophilic phosphate heads and hydrophobic fatty acid tails, they automatically arrange into bilayers - the heads face outward toward water whilst the tails hide inside.
This bilayer structure creates a selectively permeable barrier. Small, non-polar molecules can slip through the fatty acid centre, but water-soluble substances get blocked. It's like having a security system that only lets certain molecules pass.
Visual Learning: Picture phospholipids as matchsticks with magnetic heads - they'll always orient themselves with heads touching water and tails clustering together!

Cholesterol acts like a membrane thermostat, keeping cell membranes at just the right consistency. In hot conditions, it prevents excessive fluidity; in cold conditions, it stops the membrane from becoming too rigid and cracking.
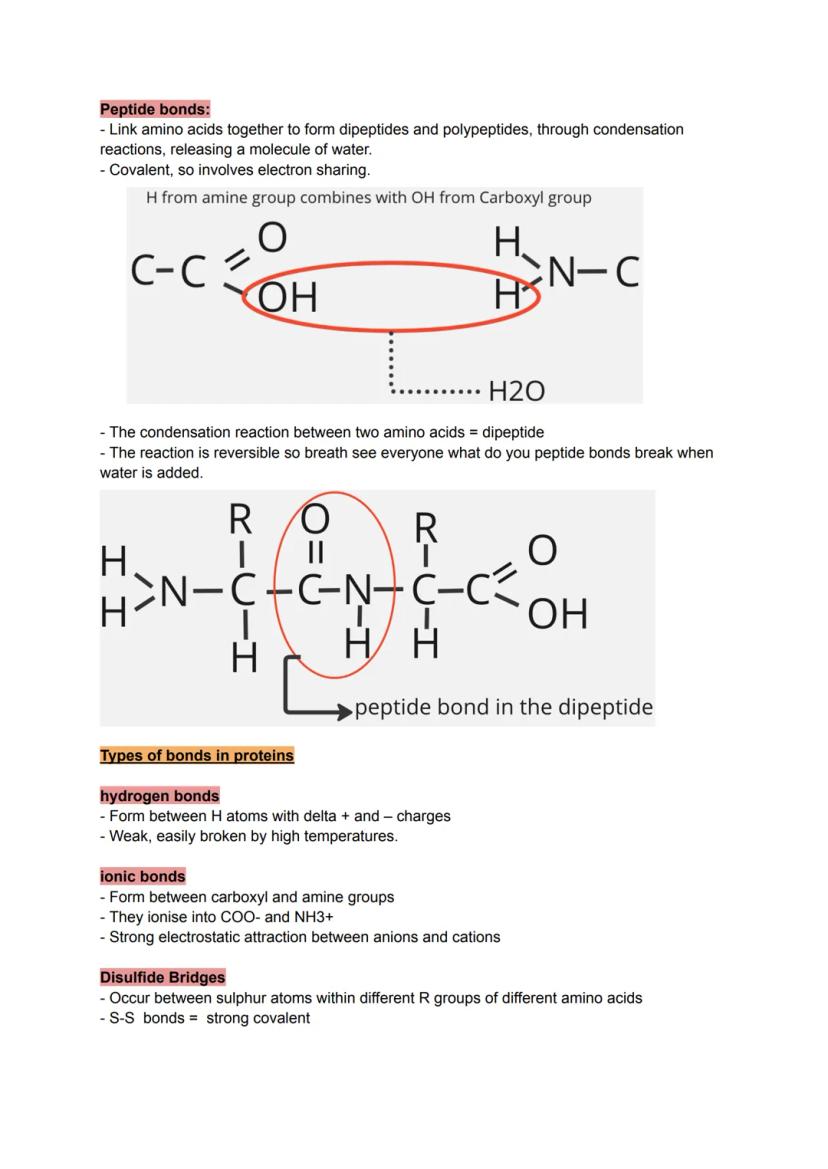
Proteins are the workhorses of your cells, built from chains of amino acids linked by peptide bonds. Each amino acid has the same basic structure except for its R group - this variable side chain gives each amino acid its unique personality.
The condensation reaction between amino acids is straightforward: the hydrogen from one amino acid's amine group combines with the hydroxyl group from another's carboxyl group, releasing water and forming a covalent peptide bond.
Study Strategy: Focus on the R groups when learning amino acids - they determine everything about how the protein will fold and function!
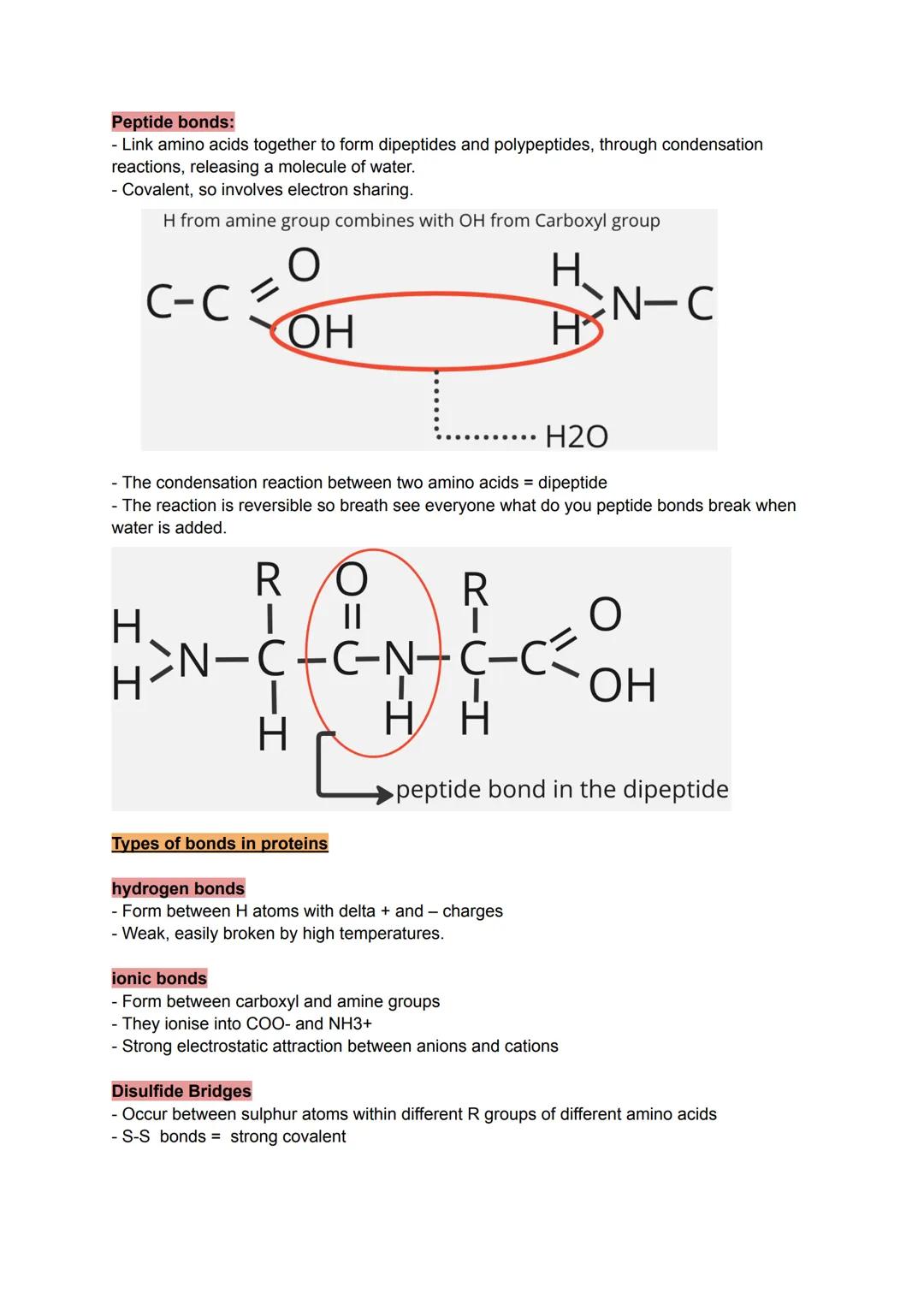
Four types of bonds hold proteins in their final shapes. Hydrogen bonds form between slightly charged atoms but break easily with heat. Ionic bonds create strong attractions between oppositely charged R groups. Disulfide bridges form the strongest covalent bonds between sulfur-containing amino acids.
Primary structure is simply the sequence of amino acids - change even one, and you change the entire protein. Secondary structure introduces basic folding patterns like alpha helixes (tight spirals) and beta pleated sheets (parallel chains with hydrogen bonds between them).
Tertiary structure creates the final 3D shape through complex interactions between R groups. Hydrophobic R groups cluster inward whilst hydrophilic ones face outward, stabilised by all four bond types working together.
Exam Essential: Temperature and pH changes break hydrogen bonds first, which is why proteins denature (lose their shape) when you cook them!

Quaternary structure only exists in proteins with multiple polypeptide chains, called subunits. Each subunit has its own tertiary structure, but they work together like different instruments in an orchestra to create the protein's final function.
The four structural levels build upon each other logically. Primary determines secondary, secondary influences tertiary, and tertiary affects how multiple chains interact in quaternary structures. It's like following a recipe where each step depends on getting the previous one right.
Understanding these levels helps explain why protein folding is so critical. A single amino acid change in the primary structure can cascade through all levels, potentially destroying the protein's function - this is what happens in genetic diseases like sickle cell anemia.
Connection Point: Computer modelling now predicts protein shapes from amino acid sequences, revolutionising drug design by showing how medicines might interact with target proteins!

Globular proteins are the specialists of the protein world - compact, spherical, and designed for specific jobs. Their hydrophilic R groups face outward for solubility, whilst hydrophobic groups cluster inside, making them perfect for transport in body fluids.
Haemoglobin exemplifies sophisticated protein design with four polypeptide chains, each carrying an iron-containing haem group. This quaternary structure allows cooperative oxygen binding - when one chain grabs oxygen, it makes the others more likely to bind too.
Insulin and pepsin show globular proteins' versatility. Insulin's compact, soluble structure lets it travel through blood to regulate glucose levels, whilst pepsin's stable tertiary structure survives stomach acid to digest other proteins.
Key Insight: Prosthetic groups like haem aren't made of amino acids but are essential for protein function - removing iron from haemoglobin makes it useless for oxygen transport!

Fibrous proteins are your body's building materials - long, strong, and perfectly designed for structural roles. Their repetitive sequences and cross-linked chains create incredible tensile strength, like biological steel cables.
Collagen forms the framework for skin, tendons, and bones. Its flexibility allows movement whilst providing strength, and its ability to bind minerals makes it essential for bone formation. Keratin varies from flexible (skin) to rigid (nails) depending on how many disulfide bridges cross-link its structure.
Elastin solves the problem of tissues that need to stretch and spring back. Found in skin, blood vessels, and lung tissue, it allows these structures to expand under pressure then return to their original size.
Real-world Application: Understanding collagen structure helps explain why vitamin C deficiency causes scurvy - without it, collagen can't form properly, leading to weak connective tissues!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
M
@iamjungkook
Ever wonder what makes your DNA different from a piece of toast? It all comes down to biological molecules - the essential building blocks that make life possible. You'll discover how simple monomers join together like LEGO bricks to create... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of biological molecules like construction sets - monomers are the individual bricks, whilst polymers are the finished structures built from identical pieces joined together. This simple concept explains how your body creates everything from energy stores to muscle fibres.
Two key reactions control this molecular construction site. Condensation reactions join monomers together by removing water molecules, like welding pieces with the loss of steam. Hydrolysis reactions do the opposite - they break apart large molecules by adding water back, which is exactly what happens when you digest food.
Carbohydrates follow the CHO formula and serve as your body's primary energy source. Monosaccharides like glucose are single sugar units that dissolve easily in blood for quick transport around your body.
Quick Tip: Remember that alpha glucose (used in starch) has hydrogen above the first carbon, whilst beta glucose (used in cellulose) has the OH group above - this tiny difference completely changes their properties!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ribose and deoxyribose might sound complicated, but they're just five-carbon sugars with a crucial difference. Ribose (in RNA) keeps all its OH groups, whilst deoxyribose (in DNA) loses one oxygen atom - making DNA more stable for long-term information storage.
When two monosaccharides join through condensation reactions, they form glycosidic bonds and create disaccharides like table sugar. These double sugars remain soluble and sweet, perfect for quick energy hits.
Polysaccharides are the heavy-duty storage units - long chains of sugars linked by glycosidic bonds. Most are insoluble due to their massive, branched structures, which makes them ideal for storing energy without affecting your cells' water balance.
Exam Focus: Each glycosidic bond formation releases one water molecule - count the bonds to work out how much water is produced in synthesis reactions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Plants face the same problem you do - they need to store excess energy for later use. Starch solves this perfectly by being completely insoluble, so it won't mess with the plant's water balance while storing massive amounts of glucose.
Amylose forms unbranched chains that coil into tight spirals, packing efficiently like a compressed spring. Its compact structure makes it brilliant for storage but slow to break down. Amylopectin takes a different approach with its branched design, allowing enzymes to attack multiple points simultaneously for faster glucose release.
The branching happens through different bond types - straight sections use 1-4 glycosidic bonds, whilst branch points use 1-6 bonds. This creates a tree-like structure that balances storage efficiency with accessibility.
Memory Trick: Amylose = "A-my-coiled" (coiled structure), Amylopectin = "A-my-branches" (branched structure)!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Glycogen is basically amylopectin on steroids - it has branches every 8-12 glucose units compared to starch's longer straight sections. This ultra-branched structure reflects animals' higher metabolic demands and need for rapid energy release during movement.
Cellulose breaks all the rules by using beta glucose, creating straight, unbranched chains where every other glucose molecule flips 180°. This alternating pattern allows adjacent molecules to form hydrogen bonds, creating rope-like microfibrils of incredible strength.
Triglycerides represent a completely different storage strategy. Made from glycerol plus three fatty acids joined by ester bonds, they pack almost twice the energy of carbohydrates. Their hydrophobic nature means they won't absorb water and swell up like a sponge.
Real-world Connection: Cellulose's strength-to-weight ratio rivals steel - that's why it's perfect for plant cell walls and why we use it to make paper!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Saturated fatty acids pack together tightly like straight pencils in a box, whilst unsaturated fatty acids have kinks from double bonds that push molecules apart. More kinks mean lower melting points - think of butter (saturated) versus olive oil (unsaturated) at room temperature.
Phospholipids are the molecular architects of cell membranes. With hydrophilic phosphate heads and hydrophobic fatty acid tails, they automatically arrange into bilayers - the heads face outward toward water whilst the tails hide inside.
This bilayer structure creates a selectively permeable barrier. Small, non-polar molecules can slip through the fatty acid centre, but water-soluble substances get blocked. It's like having a security system that only lets certain molecules pass.
Visual Learning: Picture phospholipids as matchsticks with magnetic heads - they'll always orient themselves with heads touching water and tails clustering together!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Cholesterol acts like a membrane thermostat, keeping cell membranes at just the right consistency. In hot conditions, it prevents excessive fluidity; in cold conditions, it stops the membrane from becoming too rigid and cracking.
Proteins are the workhorses of your cells, built from chains of amino acids linked by peptide bonds. Each amino acid has the same basic structure except for its R group - this variable side chain gives each amino acid its unique personality.
The condensation reaction between amino acids is straightforward: the hydrogen from one amino acid's amine group combines with the hydroxyl group from another's carboxyl group, releasing water and forming a covalent peptide bond.
Study Strategy: Focus on the R groups when learning amino acids - they determine everything about how the protein will fold and function!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Four types of bonds hold proteins in their final shapes. Hydrogen bonds form between slightly charged atoms but break easily with heat. Ionic bonds create strong attractions between oppositely charged R groups. Disulfide bridges form the strongest covalent bonds between sulfur-containing amino acids.
Primary structure is simply the sequence of amino acids - change even one, and you change the entire protein. Secondary structure introduces basic folding patterns like alpha helixes (tight spirals) and beta pleated sheets (parallel chains with hydrogen bonds between them).
Tertiary structure creates the final 3D shape through complex interactions between R groups. Hydrophobic R groups cluster inward whilst hydrophilic ones face outward, stabilised by all four bond types working together.
Exam Essential: Temperature and pH changes break hydrogen bonds first, which is why proteins denature (lose their shape) when you cook them!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Quaternary structure only exists in proteins with multiple polypeptide chains, called subunits. Each subunit has its own tertiary structure, but they work together like different instruments in an orchestra to create the protein's final function.
The four structural levels build upon each other logically. Primary determines secondary, secondary influences tertiary, and tertiary affects how multiple chains interact in quaternary structures. It's like following a recipe where each step depends on getting the previous one right.
Understanding these levels helps explain why protein folding is so critical. A single amino acid change in the primary structure can cascade through all levels, potentially destroying the protein's function - this is what happens in genetic diseases like sickle cell anemia.
Connection Point: Computer modelling now predicts protein shapes from amino acid sequences, revolutionising drug design by showing how medicines might interact with target proteins!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Globular proteins are the specialists of the protein world - compact, spherical, and designed for specific jobs. Their hydrophilic R groups face outward for solubility, whilst hydrophobic groups cluster inside, making them perfect for transport in body fluids.
Haemoglobin exemplifies sophisticated protein design with four polypeptide chains, each carrying an iron-containing haem group. This quaternary structure allows cooperative oxygen binding - when one chain grabs oxygen, it makes the others more likely to bind too.
Insulin and pepsin show globular proteins' versatility. Insulin's compact, soluble structure lets it travel through blood to regulate glucose levels, whilst pepsin's stable tertiary structure survives stomach acid to digest other proteins.
Key Insight: Prosthetic groups like haem aren't made of amino acids but are essential for protein function - removing iron from haemoglobin makes it useless for oxygen transport!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Fibrous proteins are your body's building materials - long, strong, and perfectly designed for structural roles. Their repetitive sequences and cross-linked chains create incredible tensile strength, like biological steel cables.
Collagen forms the framework for skin, tendons, and bones. Its flexibility allows movement whilst providing strength, and its ability to bind minerals makes it essential for bone formation. Keratin varies from flexible (skin) to rigid (nails) depending on how many disulfide bridges cross-link its structure.
Elastin solves the problem of tissues that need to stretch and spring back. Found in skin, blood vessels, and lung tissue, it allows these structures to expand under pressure then return to their original size.
Real-world Application: Understanding collagen structure helps explain why vitamin C deficiency causes scurvy - without it, collagen can't form properly, leading to weak connective tissues!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
12
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the four levels of protein structure: primary, secondary, tertiary, and quaternary. This detailed summary covers key concepts such as peptide bonds, hydrogen bonds, and the differences between globular and fibrous proteins. Ideal for A Level Biology students preparing for exams.
Paper 1. What is a protein? What it’s structure? What are types of bonding involved? How to test for proteins? Globular VS Fibrous. Haemoglobin VS Collagen.
Explore the intricate world of proteins with this detailed summary covering their structures, functions, and the role of amino acids. Understand primary, secondary, tertiary, and quaternary structures, along with key concepts like peptide bonds, denaturation, and enzyme functions. Ideal for AQA A-level biology students.
Explore the intricate structure of proteins, including amino acid composition, peptide bonds, and the four levels of protein structure: primary, secondary, tertiary, and quaternary. This summary is essential for OCR A Level Biology, covering key concepts such as protein functions, enzyme roles, and the significance of various bonds in maintaining protein shape.
Explore the intricate world of proteins, including their structure, functions, and the role of amino acids. This summary covers key concepts such as peptide bonds, denaturation, and the four levels of protein organization. Ideal for A-Level Biology students studying biological molecules.
Explore the key factors affecting enzyme activity, including temperature, pH, and substrate concentration. This summary covers enzyme structure, the active site, and the 'lock and key' model, providing essential insights for GCSE Biology students. Ideal for exam preparation and understanding enzyme kinetics.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user