Ever wondered how scientists conduct proper experiments or how cells... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
361
•
5 Feb 2026
•
avocado
@avocado_03
Ever wondered how scientists conduct proper experiments or how cells... Show more










Understanding variables is crucial for any biological investigation. The independent variable (IV) is what you're testing, the dependent variable (DV) is what changes as a result, and control variables (CV) are kept constant to ensure valid results.
To boost confidence in your results, you'll want to increase sample sizes in each group - this helps spot anomalies and makes your mean more accurate. Adding standard deviation calculations and statistical tests when comparing means will make your conclusions much stronger.
When working with microscopes, remember that magnification shows how many times larger an image appears compared to the real object, while resolution determines how well you can distinguish between two separate points. The key measurement conversions you'll need are: centimetres to millimetres (×10), millimetres to micrometres (×1000), and micrometres to nanometres (×1000).
Quick Tip: For better microscopic drawings, always add a scale, include a title, and avoid shading - these simple steps will improve your scientific accuracy significantly.

Staining is your best friend when examining cells under a microscope - it increases contrast, makes cells visible, and creates clearer images. Differential staining in electron microscopy can even help identify specific organelles and distinguish between different cell types.
The nucleus is where RNA synthesis happens, whilst the rough endoplasmic reticulum (RER) handles protein translation. The RER's membrane provides compartmentalisation, controls entry, and holds ribosomes in place. The Golgi apparatus then modifies and packages these proteins for transport.
Mitochondria are essential for aerobic respiration, producing the ATP needed for active transport, cell division, protein synthesis, and DNA replication. The cytoskeleton provides mechanical strength, supports cells, and helps move molecules around inside the cell.
Remember: When measuring cell structures like white blood cell nuclei, always use an eyepiece graticule calibrated with a stage micrometer, take repeat measurements, and calculate a mean for accuracy.

Water's unique properties make life possible. Its polar molecules make it an excellent solvent, allowing it to bind to various solute molecules. Hydrogen bonds hold water molecules together, and hydrogen ions help regulate pH whilst sodium ions regulate water potential.
Water's density properties are crucial for organism survival - ice is less dense than water, so it floats and insulates aquatic life below. Water's high specific heat capacity makes it an ideal habitat for organisms like amphibians.
The chemical elements in biological molecules follow clear patterns: carbohydrates (like sucrose) contain C, H, O; lipids (like cholesterol) also contain C, H, O; proteins (like insulin) contain C, H, O, N, S; and nucleic acids (like ATP) contain C, H, O, N, P. Sulfur is specifically required for protein synthesis.
Key Fact: Carbohydrates arranged by solubility from most to least: glucose, ribose, amylose, amylopectin - this order appears frequently in exam questions!

Starch exists in two forms: amylose (unbranched, insoluble) and amylopectin . Glycogen has a high proportion of 1,6-glycosidic bonds, creating a highly branched structure for rapid glucose release. Cellulose provides high tensile strength whilst remaining insoluble and flexible.
Plants store energy as starch, whilst animals use glycogen. Both are formed through condensation reactions between α-glucose monomers, but their different branching patterns suit their specific functions.
Cholesterol molecules increase membrane stability and are used to synthesise steroid hormones and bile. Triglycerides serve multiple functions: energy source for respiration, energy storage, phospholipid production, and thermal insulation. They contain ester bonds between fatty acids and glycerol.
Structure Tip: Phospholipids form bilayers because they have hydrophilic heads (which orient towards water) and hydrophobic tails (which orient away from water) - this arrangement is fundamental to all cell membranes.

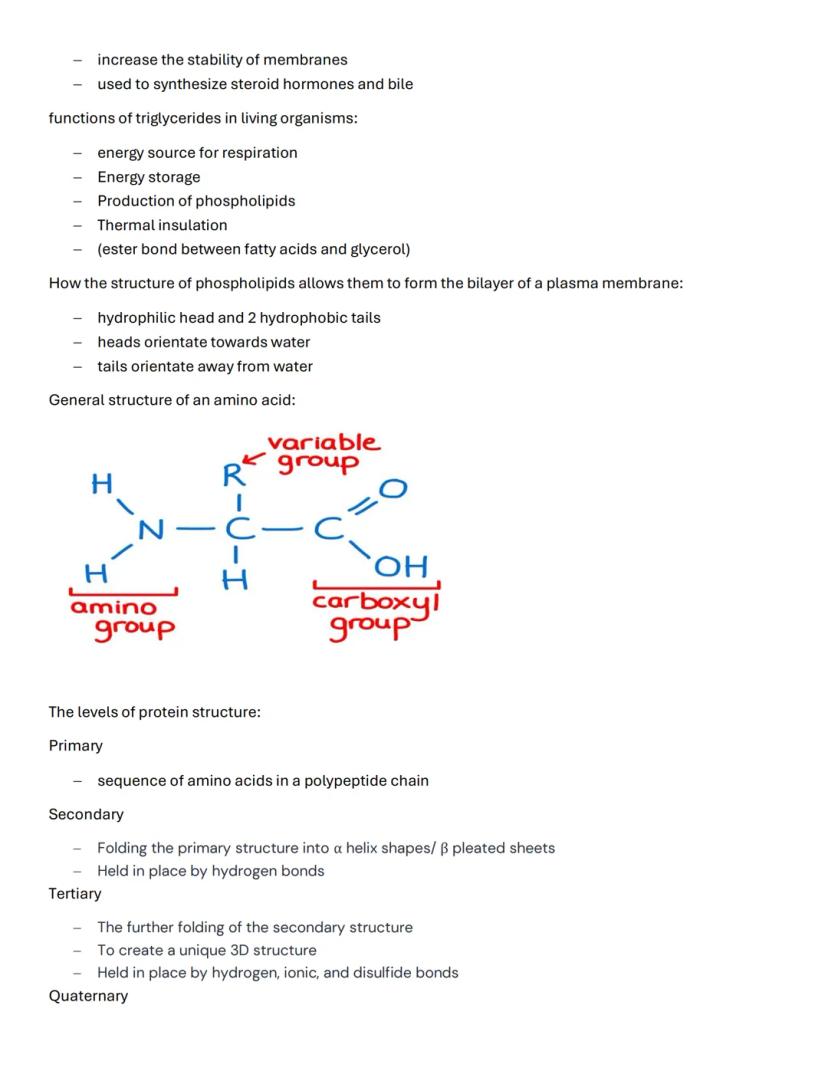
Amino acids have a standard structure: an amino group, a carboxyl group, and a variable R group attached to a central carbon. Understanding the four levels of protein structure is essential for grasping how proteins function.
Primary structure is the amino acid sequence. Secondary structure involves folding into α-helixes and β-pleated sheets held by hydrogen bonds. Tertiary structure creates unique 3D shapes through hydrogen, ionic, and disulfide bonds. Quaternary structure occurs when multiple polypeptide chains combine.
Fibrous proteins (like collagen, keratin, elastin) are strong, insoluble, and structural, with long twisted strands. Globular proteins (like haemoglobin, insulin) are soluble and metabolic, rolled into spherical shapes. Some globular proteins are conjugated proteins - they contain non-protein groups like haemoglobin's four haem groups.
Exam Essential: Remember that collagen's strength comes from its many hydrogen bonds, and dipeptides/polypeptides form through peptide bonds between amino acids.
Genes code for proteins through a precise system where each triplet codes for one amino acid. These triplets are non-overlapping and determine the primary structure (amino acid sequence) of proteins.
Transcription involves complementary base pairing to synthesise mRNA strands using RNA polymerase. Translation occurs when mRNA binds to ribosomes, tRNA brings specific amino acids, and the mRNA sequence is translated into a polypeptide chain.
Enzymes are proteins, which explains why temperature and pH changes affect their function so dramatically. Understanding this connection helps explain enzyme kinetics and denaturation.
Quick Reference: The Rf value formula (distance moved by solute ÷ distance moved by solvent) frequently appears in chromatography questions, so memorise it!

Biological membranes serve crucial roles inside cells by forming compartments and creating partially permeable barriers between organelles and cytoplasm. At cell surfaces, they enable cell signalling and provide sites for chemical reactions.
Different epithelial tissues have specialised functions: squamous epithelium provides thin surfaces for short diffusion distances (like in lungs), whilst ciliated epithelium and goblet cells work together in airways - goblet cells secrete mucus whilst cilia waft it away, with the cytoskeleton powering ciliary movement.
Single-celled organisms don't need specialised gas exchange surfaces because they have a large surface area to volume ratio, short diffusion distances, and relatively low oxygen/carbon dioxide demands.
Transport Essentials: Xylem tissue transports both water and mineral ions up plants - this dual function is often tested in exam questions about plant transport systems.

Neutrophils (white blood cells) have specialised ultrastructure for engulfing foreign cells: many lysosomes containing enzymes for breaking down pathogens, numerous mitochondria for energy, and multiple receptors on cell surface membranes for detection.
Antigens are short amino acid sequences that trigger immune responses. Antibodies bind to mast cells and T lymphocytes, with mast cells releasing histamine during allergic reactions.
Mammals store glycogen instead of glucose because it's insoluble (no effect on water potential), compact (efficient energy storage), and highly branched .
Immune System Key: Lysosomes break down foreign cells - this organelle is crucial for cellular defence mechanisms and appears in many immunity-related questions.

Natural cloning in plants occurs through runners, tubers, rhizomes, or bulbs, with new plants produced from meristem tissue. Taking cuttings involves removing stem parts, dipping in growth hormones (like auxin), and creating moist, warm environments.
Micropropagation offers advantages like rapid production of large clone numbers and year-round growing, but requires expensive equipment and skilled technicians. The process involves removing cells from meristem tissue (the explant), sterilising with ethanol, and using hormones to stimulate mitosis, creating a callus.
Artificial animal cloning uses somatic cell nuclear transfer (SCNT): removing nuclei from somatic cells, enucleating donor eggs, inserting somatic nuclei using electrofusion, and developing embryos in vitro before implantation.
Cloning Advantage: Using clones in investigations ensures genetic identity, increasing validity by controlling genetic variables - this principle underlies many experimental designs.
Microorganisms are biotechnology superstars due to their short life cycles and low energy requirements. They're used for bioremediation (removing waste from water), manufacturing drugs like insulin, and producing antibiotics (which are secondary metabolites from fungi).
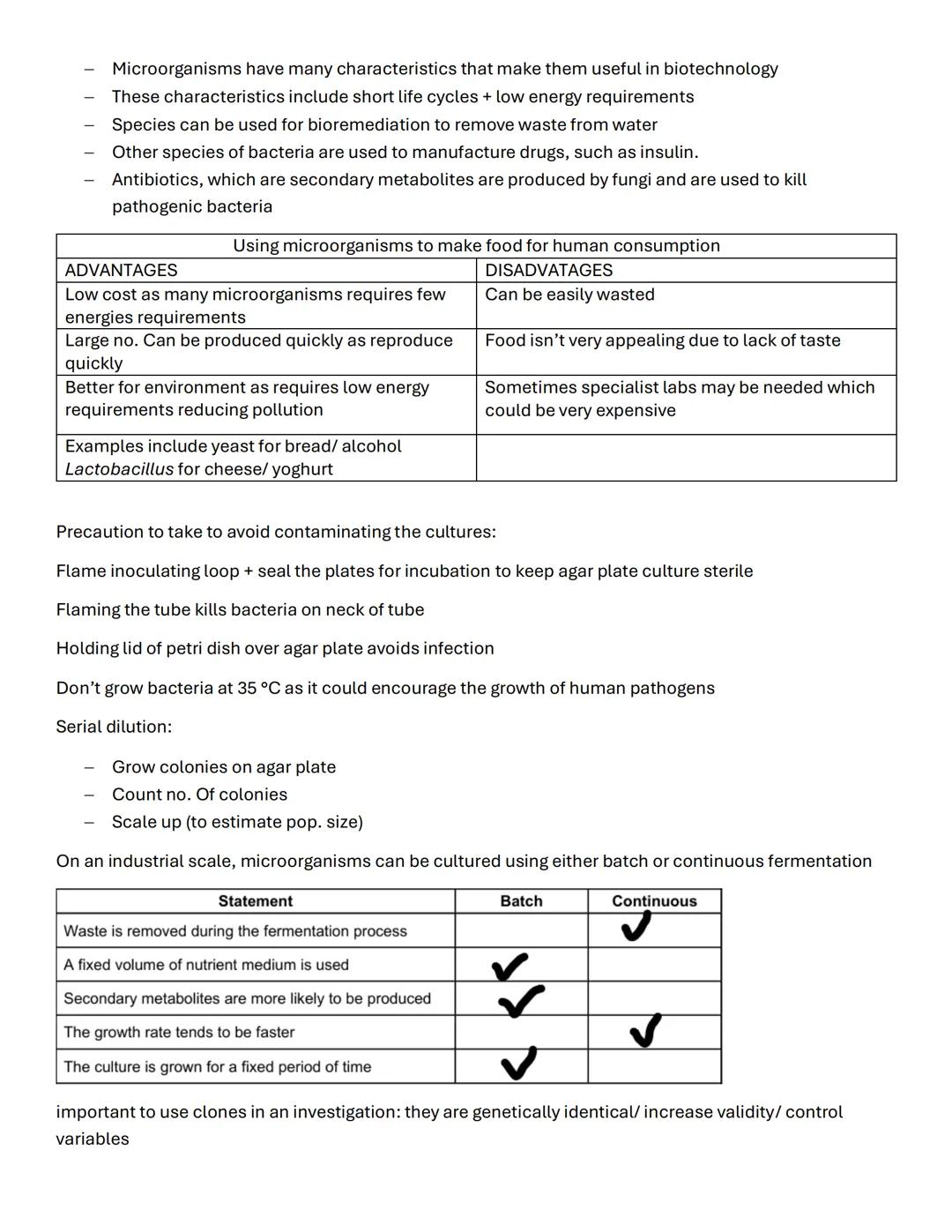
Fermentation occurs in two main types: batch fermentation uses fixed nutrient volumes for fixed time periods and produces more secondary metabolites, whilst continuous fermentation removes waste during the process and maintains faster growth rates.
Industrial microbiology requires strict contamination control: flame inoculating loops, seal plates during incubation, flame tube necks to kill bacteria, hold petri dish lids over agar plates, and avoid growing cultures at 35°C (which encourages human pathogens).
Industrial Insight: Serial dilution allows population estimation by growing colonies on agar plates, counting them, and scaling up - this technique is fundamental to industrial microbiology quality control.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
avocado
@avocado_03
Ever wondered how scientists conduct proper experiments or how cells work at the microscopic level? This comprehensive biology guide covers everything from experimental design and cell structure to biotechnology and genetic manipulation - all the essential knowledge you need for... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Understanding variables is crucial for any biological investigation. The independent variable (IV) is what you're testing, the dependent variable (DV) is what changes as a result, and control variables (CV) are kept constant to ensure valid results.
To boost confidence in your results, you'll want to increase sample sizes in each group - this helps spot anomalies and makes your mean more accurate. Adding standard deviation calculations and statistical tests when comparing means will make your conclusions much stronger.
When working with microscopes, remember that magnification shows how many times larger an image appears compared to the real object, while resolution determines how well you can distinguish between two separate points. The key measurement conversions you'll need are: centimetres to millimetres (×10), millimetres to micrometres (×1000), and micrometres to nanometres (×1000).
Quick Tip: For better microscopic drawings, always add a scale, include a title, and avoid shading - these simple steps will improve your scientific accuracy significantly.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Staining is your best friend when examining cells under a microscope - it increases contrast, makes cells visible, and creates clearer images. Differential staining in electron microscopy can even help identify specific organelles and distinguish between different cell types.
The nucleus is where RNA synthesis happens, whilst the rough endoplasmic reticulum (RER) handles protein translation. The RER's membrane provides compartmentalisation, controls entry, and holds ribosomes in place. The Golgi apparatus then modifies and packages these proteins for transport.
Mitochondria are essential for aerobic respiration, producing the ATP needed for active transport, cell division, protein synthesis, and DNA replication. The cytoskeleton provides mechanical strength, supports cells, and helps move molecules around inside the cell.
Remember: When measuring cell structures like white blood cell nuclei, always use an eyepiece graticule calibrated with a stage micrometer, take repeat measurements, and calculate a mean for accuracy.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Water's unique properties make life possible. Its polar molecules make it an excellent solvent, allowing it to bind to various solute molecules. Hydrogen bonds hold water molecules together, and hydrogen ions help regulate pH whilst sodium ions regulate water potential.
Water's density properties are crucial for organism survival - ice is less dense than water, so it floats and insulates aquatic life below. Water's high specific heat capacity makes it an ideal habitat for organisms like amphibians.
The chemical elements in biological molecules follow clear patterns: carbohydrates (like sucrose) contain C, H, O; lipids (like cholesterol) also contain C, H, O; proteins (like insulin) contain C, H, O, N, S; and nucleic acids (like ATP) contain C, H, O, N, P. Sulfur is specifically required for protein synthesis.
Key Fact: Carbohydrates arranged by solubility from most to least: glucose, ribose, amylose, amylopectin - this order appears frequently in exam questions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Starch exists in two forms: amylose (unbranched, insoluble) and amylopectin . Glycogen has a high proportion of 1,6-glycosidic bonds, creating a highly branched structure for rapid glucose release. Cellulose provides high tensile strength whilst remaining insoluble and flexible.
Plants store energy as starch, whilst animals use glycogen. Both are formed through condensation reactions between α-glucose monomers, but their different branching patterns suit their specific functions.
Cholesterol molecules increase membrane stability and are used to synthesise steroid hormones and bile. Triglycerides serve multiple functions: energy source for respiration, energy storage, phospholipid production, and thermal insulation. They contain ester bonds between fatty acids and glycerol.
Structure Tip: Phospholipids form bilayers because they have hydrophilic heads (which orient towards water) and hydrophobic tails (which orient away from water) - this arrangement is fundamental to all cell membranes.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Amino acids have a standard structure: an amino group, a carboxyl group, and a variable R group attached to a central carbon. Understanding the four levels of protein structure is essential for grasping how proteins function.
Primary structure is the amino acid sequence. Secondary structure involves folding into α-helixes and β-pleated sheets held by hydrogen bonds. Tertiary structure creates unique 3D shapes through hydrogen, ionic, and disulfide bonds. Quaternary structure occurs when multiple polypeptide chains combine.
Fibrous proteins (like collagen, keratin, elastin) are strong, insoluble, and structural, with long twisted strands. Globular proteins (like haemoglobin, insulin) are soluble and metabolic, rolled into spherical shapes. Some globular proteins are conjugated proteins - they contain non-protein groups like haemoglobin's four haem groups.
Exam Essential: Remember that collagen's strength comes from its many hydrogen bonds, and dipeptides/polypeptides form through peptide bonds between amino acids.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Genes code for proteins through a precise system where each triplet codes for one amino acid. These triplets are non-overlapping and determine the primary structure (amino acid sequence) of proteins.
Transcription involves complementary base pairing to synthesise mRNA strands using RNA polymerase. Translation occurs when mRNA binds to ribosomes, tRNA brings specific amino acids, and the mRNA sequence is translated into a polypeptide chain.
Enzymes are proteins, which explains why temperature and pH changes affect their function so dramatically. Understanding this connection helps explain enzyme kinetics and denaturation.
Quick Reference: The Rf value formula (distance moved by solute ÷ distance moved by solvent) frequently appears in chromatography questions, so memorise it!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Biological membranes serve crucial roles inside cells by forming compartments and creating partially permeable barriers between organelles and cytoplasm. At cell surfaces, they enable cell signalling and provide sites for chemical reactions.
Different epithelial tissues have specialised functions: squamous epithelium provides thin surfaces for short diffusion distances (like in lungs), whilst ciliated epithelium and goblet cells work together in airways - goblet cells secrete mucus whilst cilia waft it away, with the cytoskeleton powering ciliary movement.
Single-celled organisms don't need specialised gas exchange surfaces because they have a large surface area to volume ratio, short diffusion distances, and relatively low oxygen/carbon dioxide demands.
Transport Essentials: Xylem tissue transports both water and mineral ions up plants - this dual function is often tested in exam questions about plant transport systems.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Neutrophils (white blood cells) have specialised ultrastructure for engulfing foreign cells: many lysosomes containing enzymes for breaking down pathogens, numerous mitochondria for energy, and multiple receptors on cell surface membranes for detection.
Antigens are short amino acid sequences that trigger immune responses. Antibodies bind to mast cells and T lymphocytes, with mast cells releasing histamine during allergic reactions.
Mammals store glycogen instead of glucose because it's insoluble (no effect on water potential), compact (efficient energy storage), and highly branched .
Immune System Key: Lysosomes break down foreign cells - this organelle is crucial for cellular defence mechanisms and appears in many immunity-related questions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Natural cloning in plants occurs through runners, tubers, rhizomes, or bulbs, with new plants produced from meristem tissue. Taking cuttings involves removing stem parts, dipping in growth hormones (like auxin), and creating moist, warm environments.
Micropropagation offers advantages like rapid production of large clone numbers and year-round growing, but requires expensive equipment and skilled technicians. The process involves removing cells from meristem tissue (the explant), sterilising with ethanol, and using hormones to stimulate mitosis, creating a callus.
Artificial animal cloning uses somatic cell nuclear transfer (SCNT): removing nuclei from somatic cells, enucleating donor eggs, inserting somatic nuclei using electrofusion, and developing embryos in vitro before implantation.
Cloning Advantage: Using clones in investigations ensures genetic identity, increasing validity by controlling genetic variables - this principle underlies many experimental designs.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Microorganisms are biotechnology superstars due to their short life cycles and low energy requirements. They're used for bioremediation (removing waste from water), manufacturing drugs like insulin, and producing antibiotics (which are secondary metabolites from fungi).
Fermentation occurs in two main types: batch fermentation uses fixed nutrient volumes for fixed time periods and produces more secondary metabolites, whilst continuous fermentation removes waste during the process and maintains faster growth rates.
Industrial microbiology requires strict contamination control: flame inoculating loops, seal plates during incubation, flame tube necks to kill bacteria, hold petri dish lids over agar plates, and avoid growing cultures at 35°C (which encourages human pathogens).
Industrial Insight: Serial dilution allows population estimation by growing colonies on agar plates, counting them, and scaling up - this technique is fundamental to industrial microbiology quality control.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
7
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
OCR a level biology
Explore the fundamental concepts of protein structure, including amino acids, peptide bonds, and the four levels of protein organization. This summary covers primary, secondary, tertiary, and quaternary structures, essential for A-Level Biology students. Ideal for quick revision and understanding of how protein structure influences function.
Explore the essential concepts of protein structure, including amino acids, peptide bonds, and the four levels of protein organization. This summary covers key topics such as the primary, secondary, tertiary, and quaternary structures, along with examples like collagen and hemoglobin. Ideal for AQA A-Level Biology students seeking a comprehensive understanding of protein functions and structures.
Explore the formation and functions of triglycerides and phospholipids, including ester bonds, energy storage, and membrane structure. This summary covers key concepts such as cholesterol's role in membrane fluidity and the hydrophilic and hydrophobic properties of lipids. Ideal for students studying lipid biochemistry.
Explore the key factors affecting enzyme activity, including temperature, pH, and substrate concentration. This summary covers enzyme structure, the active site, and the 'lock and key' model, providing essential insights for GCSE Biology students. Ideal for exam preparation and understanding enzyme kinetics.
Explore the key digestive enzymes: Amylase, Protease, and Lipase. This summary covers their functions, production sites, and optimal pH levels for activity. Ideal for students studying human biology and digestive processes.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user