Ever wondered why water droplets stick together or how your... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
119
•
5 Feb 2026
•
Maja Szulczynska
@majaszulczynska
Ever wondered why water droplets stick together or how your... Show more











Water might seem simple, but it's actually a polar molecule with some pretty amazing tricks up its sleeve. The oxygen end carries a negative charge whilst the hydrogen atoms are positively charged, creating what scientists call a dipole.
When water molecules get close together, these opposite charges attract each other, forming hydrogen bonds. Think of it like molecular magnets - individually weak, but together they create a strong lattice framework that gives water its unique properties.
This attraction between water molecules is called cohesion, and it's absolutely crucial for life. It's what allows water to travel up tall trees and helps create that bouncy surface tension you see on ponds.
Key Point: Water's polar nature makes it an excellent solvent - the positive and negative parts attract other charged particles like ions and polar molecules (such as glucose), allowing them to dissolve easily.

Water has some incredible properties that make it perfect for supporting life. Its high specific heat capacity means it takes loads of energy to change its temperature, keeping aquatic environments stable and allowing enzymes to work efficiently.
The high latent heat of vaporisation makes water brilliant for cooling - that's why sweating works so well! When water evaporates from your skin, it takes heat energy with it, cooling you down effectively.
Water serves as the body's ultimate transport medium. Blood is mostly water and carries dissolved substances around your body, whilst in plants, minerals dissolved in water travel from roots to leaves through the xylem.
Fascinating Fact: Water is less dense when frozen, so ice floats! This insulates the water below, keeping aquatic life safe during winter whilst allowing sunlight through for photosynthesis.

Carbohydrates are organic molecules made from carbon, hydrogen, and oxygen that serve as life's main energy currency. They come in three sizes: monosaccharides (single units), disaccharides (two units), and polysaccharides (many units).
Monosaccharides are grouped by their carbon count - trioses (3 carbons) are vital for respiration and photosynthesis, pentoses (5 carbons) form the backbone of DNA and RNA, whilst hexoses (6 carbons) like glucose provide energy for cellular respiration.
The star of the show is glucose, which exists in two forms: alpha and beta. This might seem like a small difference, but it completely changes how glucose molecules can link together to form larger carbohydrates.
Remember: When glucose bonds are broken during respiration, the released energy is captured to make ATP - your cells' energy currency.
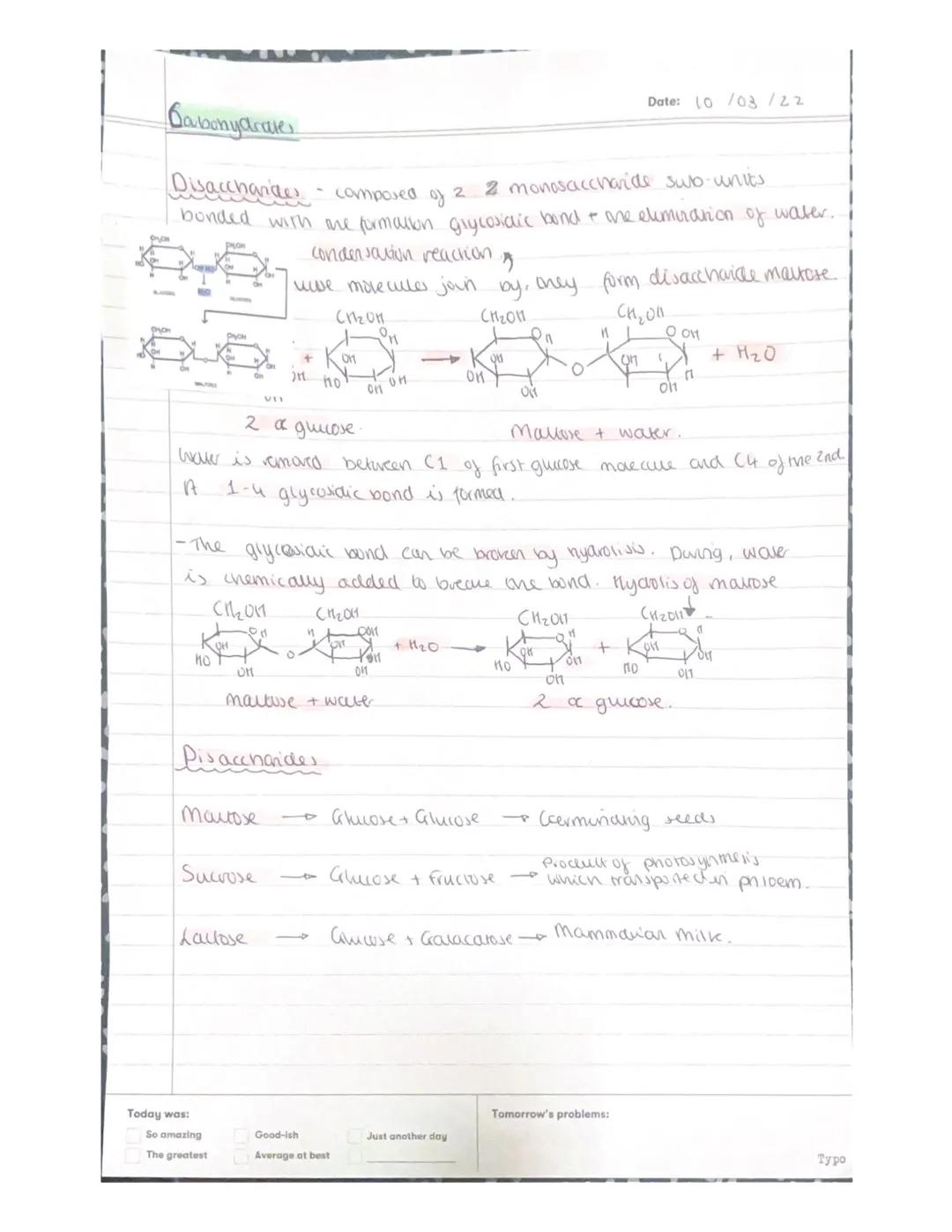
Disaccharides form when two monosaccharides join together through a condensation reaction, eliminating water and creating a glycosidic bond. It's like molecular handholding that creates something entirely new.
Maltose forms when two alpha-glucose molecules join with an α(1-4) glycosidic bond - you'll find this in germinating seeds. The bond forms between carbon 1 of the first glucose and carbon 4 of the second.
This process is completely reversible through hydrolysis - just add water and the bond breaks, splitting maltose back into two glucose molecules. Other important disaccharides include sucrose found in plants, and lactose in mammalian milk.
Test Tip: Remember that condensation removes water to form bonds, whilst hydrolysis adds water to break them - opposite processes that are fundamental to biology.

There are two types of sugars you can test for: reducing sugars and non-reducing sugars. Reducing sugars (like glucose and fructose) donate electrons in chemical reactions, whilst non-reducing sugars (like sucrose) don't.
Benedict's test is your go-to method for detecting reducing sugars. Heat equal volumes of Benedict's reagent and your test solution to 100°C. If reducing sugars are present, you'll see a colour change from blue through green, yellow, and orange to a brick-red precipitate.
For non-reducing sugars like sucrose, you need an extra step. First, break them down by heating with hydrochloric acid, then add alkali to neutralise before doing the Benedict's test. If it turns red, non-reducing sugars were originally present.
Lab Success: The intensity of the colour change in Benedict's test indicates the concentration of reducing sugars - the redder it gets, the more sugar is present.

Starch is how plants store glucose, found in high concentrations in seeds and storage organs like potato tubers. It's made of two polymers: amylose and amylopectin, both built from alpha-glucose molecules.
Amylose forms long, unbranched chains with α(1-4) glycosidic bonds, creating a linear molecule that coils into a helix. Amylopectin has the same backbone but includes α(1-6) bonds every 25-30 glucose units, creating branch points.
Glycogen is the animal equivalent of starch, serving as our main energy store in the liver and muscles. It has the same bonding pattern as amylopectin but with much shorter chains and more frequent branching, making it more compact and efficient.
Smart Design: The branched structure of glycogen and amylopectin provides more sites for enzymes to attack, allowing for rapid glucose release when energy is needed quickly.

Cellulose is the most abundant organic molecule on Earth, forming the structural framework of plant cell walls. Unlike starch, it's made from beta-glucose units joined by β(1-4) glycosidic bonds.
The beta linkage rotates adjacent glucose molecules by 180°, allowing hydrogen bonds to form between parallel chains. This creates incredibly strong microfibrils - bundles of 60-70 cellulose molecules tightly cross-linked together.
Chitin is similar to cellulose but found in insect exoskeletons and fungal cell walls. It has amino acid groups attached, making it a heteropolysaccharide that's strong, waterproof, and lightweight - perfect biological armour.
Engineering Marvel: Cell walls have several layers of fibres running at different angles, creating a laminated structure that's incredibly strong yet still permeable to water and solutes.

Lipids are non-polar molecules made of carbon, hydrogen, and oxygen that won't dissolve in water but love organic solvents like ethanol. The most common type is triglycerides - the fats and oils in your diet.
Fatty acids are long-chain carboxylic acids, usually 14-22 carbons long. They can be saturated (no double bonds), monounsaturated (one double bond), or polyunsaturated (multiple double bonds). More double bonds mean lower melting points.
Triglycerides form when glycerol (a type of alcohol) joins with three fatty acids through condensation reactions, creating ester bonds and releasing three water molecules. They're perfect for long-term energy storage, insulation, and waterproofing.
Health Connection: Understanding saturated vs unsaturated fats helps explain why some fats are solid at room temperature (butter) whilst others are liquid (olive oil).

The difference between saturated and unsaturated fatty acids lies in their chemical bonds. Saturated fats have no double bonds between carbon atoms, meaning they're "saturated" with hydrogen atoms and tend to be solid at room temperature.
Unsaturated fatty acids contain one or more C=C double bonds, which creates kinks in the molecular chain. These kinks prevent the molecules from packing tightly together, making them liquid at room temperature - that's why most plant oils are liquid.
Animal fats tend to be saturated, whilst plant oils are usually unsaturated. This has major health implications - diets high in saturated fats are linked to heart disease, whilst unsaturated fats (especially from sources like olive oil) are generally healthier.
Memory Trick: Think "saturated = solid" and "unsaturated = usually liquid" to remember which fats are which at room temperature.

Heart disease primarily results from fatty deposits in coronary arteries (atherosclerosis) and high blood pressure. Diets consistently high in saturated fats, smoking, lack of exercise, and ageing all contribute to this process.
When you digest food, lipids and proteins combine to form lipoproteins that travel through your bloodstream. These can deposit on the smooth inner walls of arteries, gradually building up atherosclerotic plaques.
As these fatty deposits accumulate, they reduce the available space for blood flow. If a plaque completely blocks an artery supplying the heart muscle, it causes a myocardial infarction - commonly known as a heart attack.
Prevention Focus: Understanding how dietary fats affect your cardiovascular system empowers you to make informed choices about nutrition and lifestyle for long-term health.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Maja Szulczynska
@majaszulczynska
Ever wondered why water droplets stick together or how your body stores energy for later use? This guide breaks down the fascinating world of biological molecules - from water's incredible properties that make life possible, to carbohydrates that fuel your... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Water might seem simple, but it's actually a polar molecule with some pretty amazing tricks up its sleeve. The oxygen end carries a negative charge whilst the hydrogen atoms are positively charged, creating what scientists call a dipole.
When water molecules get close together, these opposite charges attract each other, forming hydrogen bonds. Think of it like molecular magnets - individually weak, but together they create a strong lattice framework that gives water its unique properties.
This attraction between water molecules is called cohesion, and it's absolutely crucial for life. It's what allows water to travel up tall trees and helps create that bouncy surface tension you see on ponds.
Key Point: Water's polar nature makes it an excellent solvent - the positive and negative parts attract other charged particles like ions and polar molecules (such as glucose), allowing them to dissolve easily.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Water has some incredible properties that make it perfect for supporting life. Its high specific heat capacity means it takes loads of energy to change its temperature, keeping aquatic environments stable and allowing enzymes to work efficiently.
The high latent heat of vaporisation makes water brilliant for cooling - that's why sweating works so well! When water evaporates from your skin, it takes heat energy with it, cooling you down effectively.
Water serves as the body's ultimate transport medium. Blood is mostly water and carries dissolved substances around your body, whilst in plants, minerals dissolved in water travel from roots to leaves through the xylem.
Fascinating Fact: Water is less dense when frozen, so ice floats! This insulates the water below, keeping aquatic life safe during winter whilst allowing sunlight through for photosynthesis.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Carbohydrates are organic molecules made from carbon, hydrogen, and oxygen that serve as life's main energy currency. They come in three sizes: monosaccharides (single units), disaccharides (two units), and polysaccharides (many units).
Monosaccharides are grouped by their carbon count - trioses (3 carbons) are vital for respiration and photosynthesis, pentoses (5 carbons) form the backbone of DNA and RNA, whilst hexoses (6 carbons) like glucose provide energy for cellular respiration.
The star of the show is glucose, which exists in two forms: alpha and beta. This might seem like a small difference, but it completely changes how glucose molecules can link together to form larger carbohydrates.
Remember: When glucose bonds are broken during respiration, the released energy is captured to make ATP - your cells' energy currency.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Disaccharides form when two monosaccharides join together through a condensation reaction, eliminating water and creating a glycosidic bond. It's like molecular handholding that creates something entirely new.
Maltose forms when two alpha-glucose molecules join with an α(1-4) glycosidic bond - you'll find this in germinating seeds. The bond forms between carbon 1 of the first glucose and carbon 4 of the second.
This process is completely reversible through hydrolysis - just add water and the bond breaks, splitting maltose back into two glucose molecules. Other important disaccharides include sucrose found in plants, and lactose in mammalian milk.
Test Tip: Remember that condensation removes water to form bonds, whilst hydrolysis adds water to break them - opposite processes that are fundamental to biology.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
There are two types of sugars you can test for: reducing sugars and non-reducing sugars. Reducing sugars (like glucose and fructose) donate electrons in chemical reactions, whilst non-reducing sugars (like sucrose) don't.
Benedict's test is your go-to method for detecting reducing sugars. Heat equal volumes of Benedict's reagent and your test solution to 100°C. If reducing sugars are present, you'll see a colour change from blue through green, yellow, and orange to a brick-red precipitate.
For non-reducing sugars like sucrose, you need an extra step. First, break them down by heating with hydrochloric acid, then add alkali to neutralise before doing the Benedict's test. If it turns red, non-reducing sugars were originally present.
Lab Success: The intensity of the colour change in Benedict's test indicates the concentration of reducing sugars - the redder it gets, the more sugar is present.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Starch is how plants store glucose, found in high concentrations in seeds and storage organs like potato tubers. It's made of two polymers: amylose and amylopectin, both built from alpha-glucose molecules.
Amylose forms long, unbranched chains with α(1-4) glycosidic bonds, creating a linear molecule that coils into a helix. Amylopectin has the same backbone but includes α(1-6) bonds every 25-30 glucose units, creating branch points.
Glycogen is the animal equivalent of starch, serving as our main energy store in the liver and muscles. It has the same bonding pattern as amylopectin but with much shorter chains and more frequent branching, making it more compact and efficient.
Smart Design: The branched structure of glycogen and amylopectin provides more sites for enzymes to attack, allowing for rapid glucose release when energy is needed quickly.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Cellulose is the most abundant organic molecule on Earth, forming the structural framework of plant cell walls. Unlike starch, it's made from beta-glucose units joined by β(1-4) glycosidic bonds.
The beta linkage rotates adjacent glucose molecules by 180°, allowing hydrogen bonds to form between parallel chains. This creates incredibly strong microfibrils - bundles of 60-70 cellulose molecules tightly cross-linked together.
Chitin is similar to cellulose but found in insect exoskeletons and fungal cell walls. It has amino acid groups attached, making it a heteropolysaccharide that's strong, waterproof, and lightweight - perfect biological armour.
Engineering Marvel: Cell walls have several layers of fibres running at different angles, creating a laminated structure that's incredibly strong yet still permeable to water and solutes.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Lipids are non-polar molecules made of carbon, hydrogen, and oxygen that won't dissolve in water but love organic solvents like ethanol. The most common type is triglycerides - the fats and oils in your diet.
Fatty acids are long-chain carboxylic acids, usually 14-22 carbons long. They can be saturated (no double bonds), monounsaturated (one double bond), or polyunsaturated (multiple double bonds). More double bonds mean lower melting points.
Triglycerides form when glycerol (a type of alcohol) joins with three fatty acids through condensation reactions, creating ester bonds and releasing three water molecules. They're perfect for long-term energy storage, insulation, and waterproofing.
Health Connection: Understanding saturated vs unsaturated fats helps explain why some fats are solid at room temperature (butter) whilst others are liquid (olive oil).

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The difference between saturated and unsaturated fatty acids lies in their chemical bonds. Saturated fats have no double bonds between carbon atoms, meaning they're "saturated" with hydrogen atoms and tend to be solid at room temperature.
Unsaturated fatty acids contain one or more C=C double bonds, which creates kinks in the molecular chain. These kinks prevent the molecules from packing tightly together, making them liquid at room temperature - that's why most plant oils are liquid.
Animal fats tend to be saturated, whilst plant oils are usually unsaturated. This has major health implications - diets high in saturated fats are linked to heart disease, whilst unsaturated fats (especially from sources like olive oil) are generally healthier.
Memory Trick: Think "saturated = solid" and "unsaturated = usually liquid" to remember which fats are which at room temperature.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Heart disease primarily results from fatty deposits in coronary arteries (atherosclerosis) and high blood pressure. Diets consistently high in saturated fats, smoking, lack of exercise, and ageing all contribute to this process.
When you digest food, lipids and proteins combine to form lipoproteins that travel through your bloodstream. These can deposit on the smooth inner walls of arteries, gradually building up atherosclerotic plaques.
As these fatty deposits accumulate, they reduce the available space for blood flow. If a plaque completely blocks an artery supplying the heart muscle, it causes a myocardial infarction - commonly known as a heart attack.
Prevention Focus: Understanding how dietary fats affect your cardiovascular system empowers you to make informed choices about nutrition and lifestyle for long-term health.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
4
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the fundamentals of carbohydrates, including monosaccharides, disaccharides, and polysaccharides. This summary covers key concepts such as monomers, condensation, and hydrolysis, with clear definitions and examples like glucose, maltose, and starch. Ideal for AS level students seeking to understand carbohydrate chemistry.
Explore comprehensive revision notes on biological molecules, including carbohydrates, lipids, proteins, and nucleic acids. Understand key concepts such as enzyme-substrate complexes, DNA replication, and the properties of ATP. Ideal for AS/A Level Biology students preparing for exams.
Explore the fundamentals of monomers and polymers in biological molecules. This summary covers key concepts such as the structure of glucose, types of carbohydrates, and the processes of condensation and hydrolysis. Ideal for Year 12 Biology students preparing for exams.
Explore the essential concepts of biological molecules, including carbohydrates, proteins, and lipids. This summary covers the structure and function of key macromolecules, condensation and hydrolysis reactions, and the role of hydrogen bonds in biological systems. Ideal for students preparing for exams or seeking a comprehensive understanding of biomolecules.
Explore the essential concepts of biological molecules, including the roles of water, monomers, and polymers. This summary covers carbohydrates, their types (monosaccharides, disaccharides, polysaccharides), and key processes like condensation and hydrolysis. Ideal for biology students seeking to grasp the fundamentals of biomolecules and their significance in metabolic reactions.
simplified notes for quick recall
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user