The Atomic Structure mind map GCSEprovides a comprehensive overview... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
422
•
9 Jan 2026
•
Tanishtha Malik
@tanishthamalik_ttxk
The Atomic Structure mind map GCSEprovides a comprehensive overview... Show more



This page of the BONDING STRUCTURE and the PROPERTIES of matter mind map focuses on metal reactivity and displacement reactions, crucial concepts in GCSE Chemistry.
The mind map covers:
Example: Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s) This reaction demonstrates how zinc, being higher in the reactivity series, can displace copper from a solution of its salts.
Vocabulary: Reactivity series - A list of elements in order of reactivity, with the most reactive at the top.
Reactions of metals with water and steam: The mind map illustrates these reactions with general equations and specific examples.
The reactivity series: A mnemonic is provided to help remember the order of metals in the reactivity series.
Highlight: The mnemonic "Please Stop Calling Careless Zebras" can be used to remember the order: Potassium, Sodium, Calcium, Magnesium, Aluminium, (Carbon), Zinc, Iron.
This section of the Chemistry mind maps GCSE provides valuable information for understanding Halogen displacement reactions examples and Group 7 displacement reactions practical experiments. It's an excellent resource for students preparing for questions on Displacement reactions halogens colour changes and Halogen displacement reaction equation problems.

This section of the Chemistry Paper 2 mind maps focuses on the formation of salts through various chemical reactions. It provides essential information for understanding acid-base reactions and salt formation.
Key points covered include:
Example: Acid + Metal → Salt + Hydrogen
Example: CuO + H₂SO₄ → CuSO₄ + H₂O
Vocabulary: Neutralization - A reaction between an acid and a base to produce a salt and water.
Definition: Salt - A substance formed when the hydrogen ions in an acid are replaced by either metal or ammonium ions.
This section of the Chemistry mind maps GCSE is particularly useful for students working on Displacement reaction Worksheet with answers and studying Displacement reactions of halogens with other halide ions. It provides a comprehensive overview of salt formation, which is crucial for understanding Displacement reaction of halogens experiment and related topics in GCSE Chemistry.

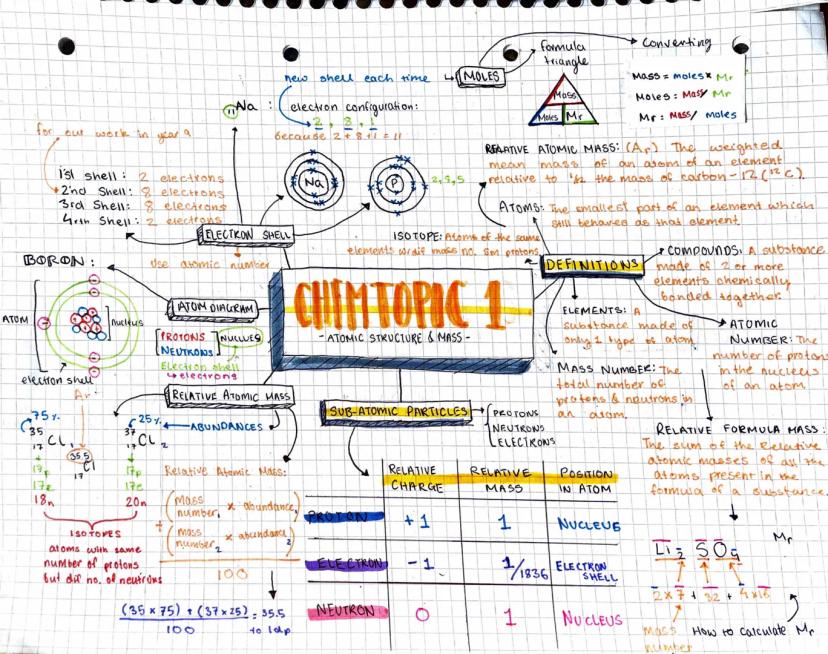
The Atomic Structure and Periodic Table mind map presents a detailed exploration of atomic structure and related concepts.
Definition: An atom is the smallest part of an element that still behaves as that element.
The mind map covers the following key areas:
Subatomic particles: protons, neutrons, and electrons, including their relative charges and masses.
Electron shell configuration: The map illustrates how electron shells are filled, with the first shell holding 2 electrons, and subsequent shells following the 2, 8, 8, 18 pattern.
Example: The electron configuration for boron is shown as 2,3, indicating 2 electrons in the first shell and 3 in the second.
Isotopes: The concept of isotopes is explained, highlighting that they are atoms of the same element with different numbers of neutrons.
Relative atomic mass: The calculation method for relative atomic mass is demonstrated using the abundances of different isotopes.
Highlight: The relative atomic mass calculation is shown for chlorine: (35 x 75) + (37 x 25) / 100 = 35.5
This comprehensive Chemistry mind maps GCSE resource provides students with a clear visual representation of atomic structure, making it an excellent study aid for A level Chemistry electron configuration questions and related topics.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Tanishtha Malik
@tanishthamalik_ttxk
The Atomic Structure mind map GCSE provides a comprehensive overview of atomic structure, chemical reactions, and salt formation. It covers key concepts in Chemistry Paper 1 mind map and Chemistry paper 2 mind maps, including electron configuration, isotopes, and... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This page of the BONDING STRUCTURE and the PROPERTIES of matter mind map focuses on metal reactivity and displacement reactions, crucial concepts in GCSE Chemistry.
The mind map covers:
Example: Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s) This reaction demonstrates how zinc, being higher in the reactivity series, can displace copper from a solution of its salts.
Vocabulary: Reactivity series - A list of elements in order of reactivity, with the most reactive at the top.
Reactions of metals with water and steam: The mind map illustrates these reactions with general equations and specific examples.
The reactivity series: A mnemonic is provided to help remember the order of metals in the reactivity series.
Highlight: The mnemonic "Please Stop Calling Careless Zebras" can be used to remember the order: Potassium, Sodium, Calcium, Magnesium, Aluminium, (Carbon), Zinc, Iron.
This section of the Chemistry mind maps GCSE provides valuable information for understanding Halogen displacement reactions examples and Group 7 displacement reactions practical experiments. It's an excellent resource for students preparing for questions on Displacement reactions halogens colour changes and Halogen displacement reaction equation problems.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This section of the Chemistry Paper 2 mind maps focuses on the formation of salts through various chemical reactions. It provides essential information for understanding acid-base reactions and salt formation.
Key points covered include:
Example: Acid + Metal → Salt + Hydrogen
Example: CuO + H₂SO₄ → CuSO₄ + H₂O
Vocabulary: Neutralization - A reaction between an acid and a base to produce a salt and water.
Definition: Salt - A substance formed when the hydrogen ions in an acid are replaced by either metal or ammonium ions.
This section of the Chemistry mind maps GCSE is particularly useful for students working on Displacement reaction Worksheet with answers and studying Displacement reactions of halogens with other halide ions. It provides a comprehensive overview of salt formation, which is crucial for understanding Displacement reaction of halogens experiment and related topics in GCSE Chemistry.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The Atomic Structure and Periodic Table mind map presents a detailed exploration of atomic structure and related concepts.
Definition: An atom is the smallest part of an element that still behaves as that element.
The mind map covers the following key areas:
Subatomic particles: protons, neutrons, and electrons, including their relative charges and masses.
Electron shell configuration: The map illustrates how electron shells are filled, with the first shell holding 2 electrons, and subsequent shells following the 2, 8, 8, 18 pattern.
Example: The electron configuration for boron is shown as 2,3, indicating 2 electrons in the first shell and 3 in the second.
Isotopes: The concept of isotopes is explained, highlighting that they are atoms of the same element with different numbers of neutrons.
Relative atomic mass: The calculation method for relative atomic mass is demonstrated using the abundances of different isotopes.
Highlight: The relative atomic mass calculation is shown for chlorine: (35 x 75) + (37 x 25) / 100 = 35.5
This comprehensive Chemistry mind maps GCSE resource provides students with a clear visual representation of atomic structure, making it an excellent study aid for A level Chemistry electron configuration questions and related topics.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
50
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the reactivity of metals, including the reactivity series, oxidation and reduction processes, and reactions with acids. This summary covers key concepts such as displacement reactions and neutralization, providing essential insights for chemistry students.
Explore the reactivity of metals through redox reactions, displacement reactions, and half equations. This summary covers key concepts such as alkali metals, oxidation, and reduction, tailored for Edexcel Chemistry Paper 1. Understand how more reactive metals displace less reactive ones and the significance of ionic equations in chemical reactions.
Explore the properties and reactivity of Group 1 (Alkali Metals), Group 0 (Noble Gases), Group 7 (Halogens), and Transition Metals. This summary covers key characteristics, reactions, and trends within the periodic table, making it essential for GCSE Chemistry students. Understand how these groups compare and their significance in chemical reactions.
Explore the fundamentals of acid-base reactions, including neutralization, titrations, and the formation of salts. This summary covers key concepts such as pH, redox reactions, and the reactivity series, providing essential insights for AQA GCSE Chemistry students. Ideal for exam preparation and understanding chemical changes.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user