Matter is made up of tiny particles that behave differently... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
1,097
•
12 Feb 2026
•
TheOnionBhaji
@theonionbhaji7
Matter is made up of tiny particles that behave differently... Show more







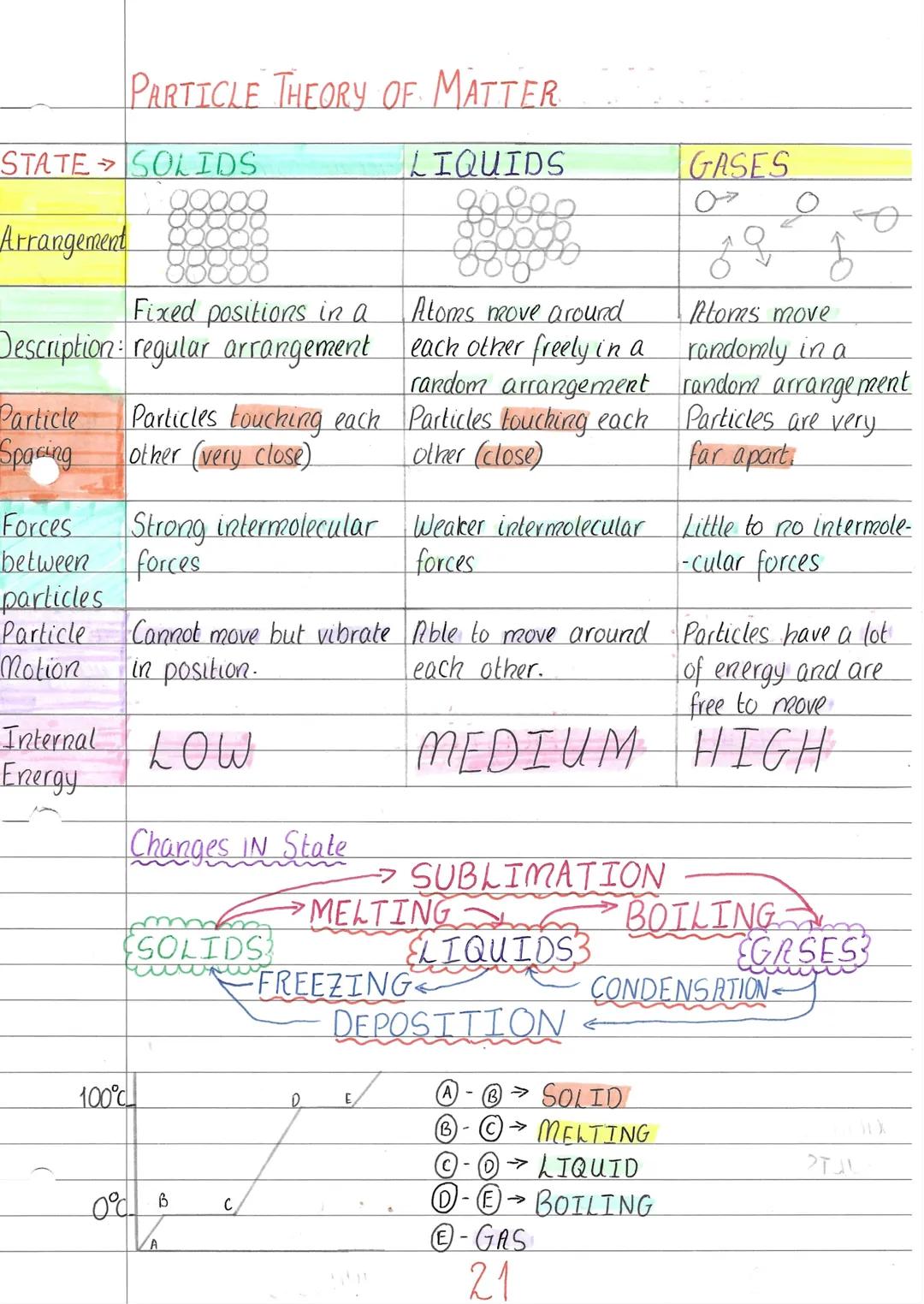
Ever wondered why ice is hard but steam floats away? It's all about how particles are arranged and how much energy they have. In solids, particles are packed tightly in fixed positions, only vibrating on the spot like people standing shoulder-to-shoulder in a crowded lift.
Liquids have particles that are still close together but can slide around each other - imagine a busy dance floor where people move but stay relatively close. The particles have more energy than in solids, so the intermolecular forces holding them together are weaker.
Gases are completely different - particles zoom around randomly with loads of space between them, like footballs being kicked around an enormous pitch. They have high internal energy and very weak forces between particles. When you heat or cool materials, you can make them change state through processes like melting, boiling, freezing, and even sublimation (solid straight to gas).
Quick Tip: Remember that temperature stays constant during state changes - all the energy goes into breaking or forming bonds between particles!

Different materials need different amounts of energy to heat up - that's why a metal spoon gets scorching hot in your tea whilst a wooden one stays cool. Specific heat capacity tells us exactly how much energy we need to raise 1kg of a material by 1°C.
The key formula you'll need is ΔE = mcΔθ, where ΔE is the change in thermal energy, m is mass, c is specific heat capacity, and Δθ is the temperature change. Water has a particularly high specific heat capacity , which is why it takes ages to boil a kettle!
In the required practical, you'll heat a metal block with an electric heater whilst measuring the current, voltage, and temperature change. The tricky bit is that the block keeps heating up even after you switch off the heater, so you need to record the highest temperature it reaches.
Safety is crucial here - those heaters get dangerously hot, so never touch them directly. Always wrap your block in cotton wool for insulation to get accurate results.
Exam Tip: Energy from the heater equals current × time × voltage . Use this to find the total energy, then rearrange the main formula to find specific heat capacity!

Here's something mind-blowing: when ice melts or water boils, the temperature stays exactly the same even though you're still adding energy. That energy isn't disappearing - it's being used to break the bonds between particles instead of making them move faster.
Specific latent heat is the energy needed to change 1kg of a material from one state to another without changing temperature. Every material has two types: latent heat of fusion and latent heat of vaporisation .
The formula is simpler than specific heat capacity: E = mL, where L is the specific latent heat. You can spot these energy changes on heating curves - they're the flat horizontal bits where temperature stays constant whilst the material changes state.
For water, the latent heat of vaporisation is absolutely massive compared to fusion . This explains why steam burns are so much worse than boiling water - that steam releases enormous amounts of energy when it condenses on your skin.
Memory Trick: Think "L for state Lines" - latent heat happens along the horizontal lines on heating/cooling curves!
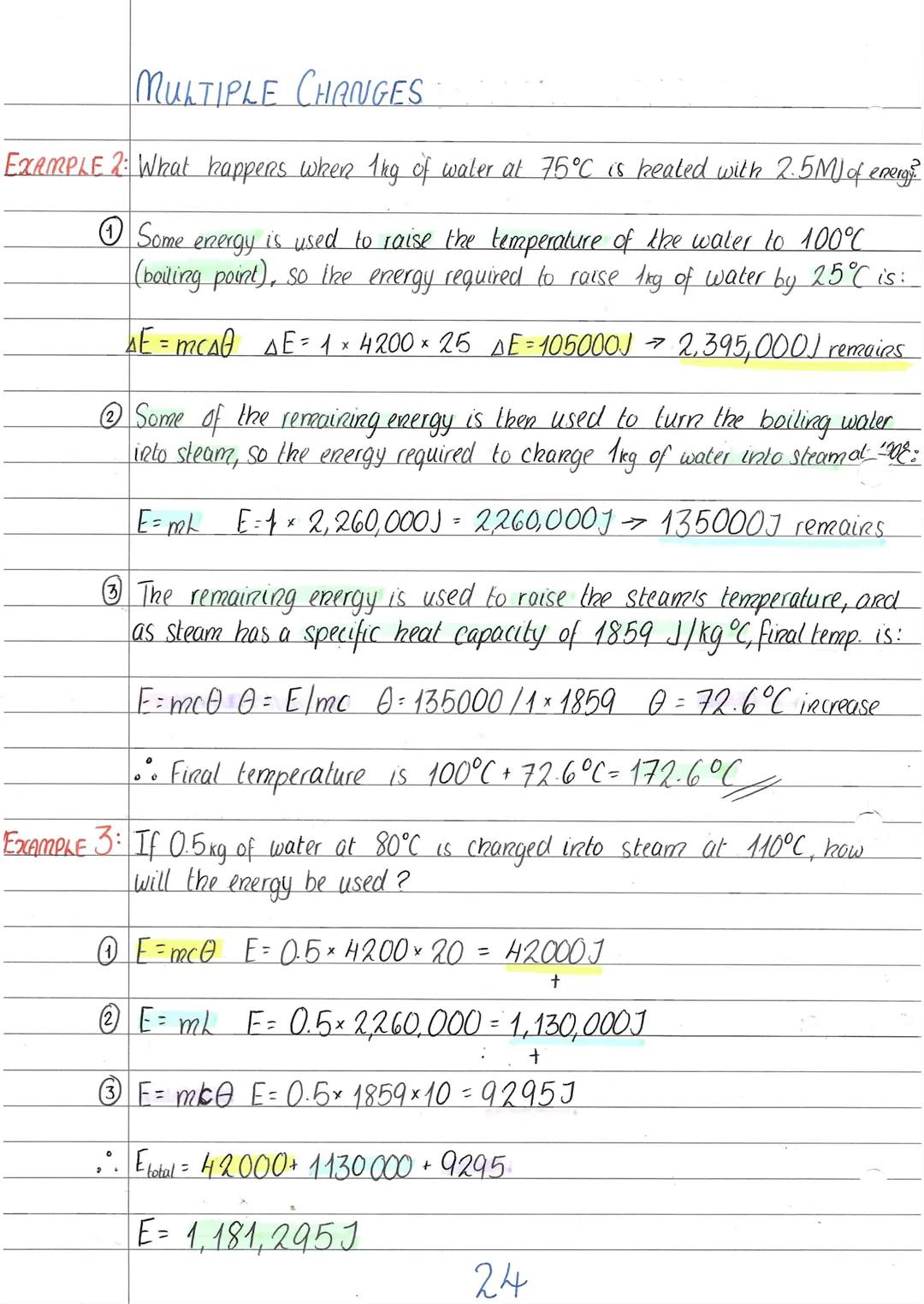
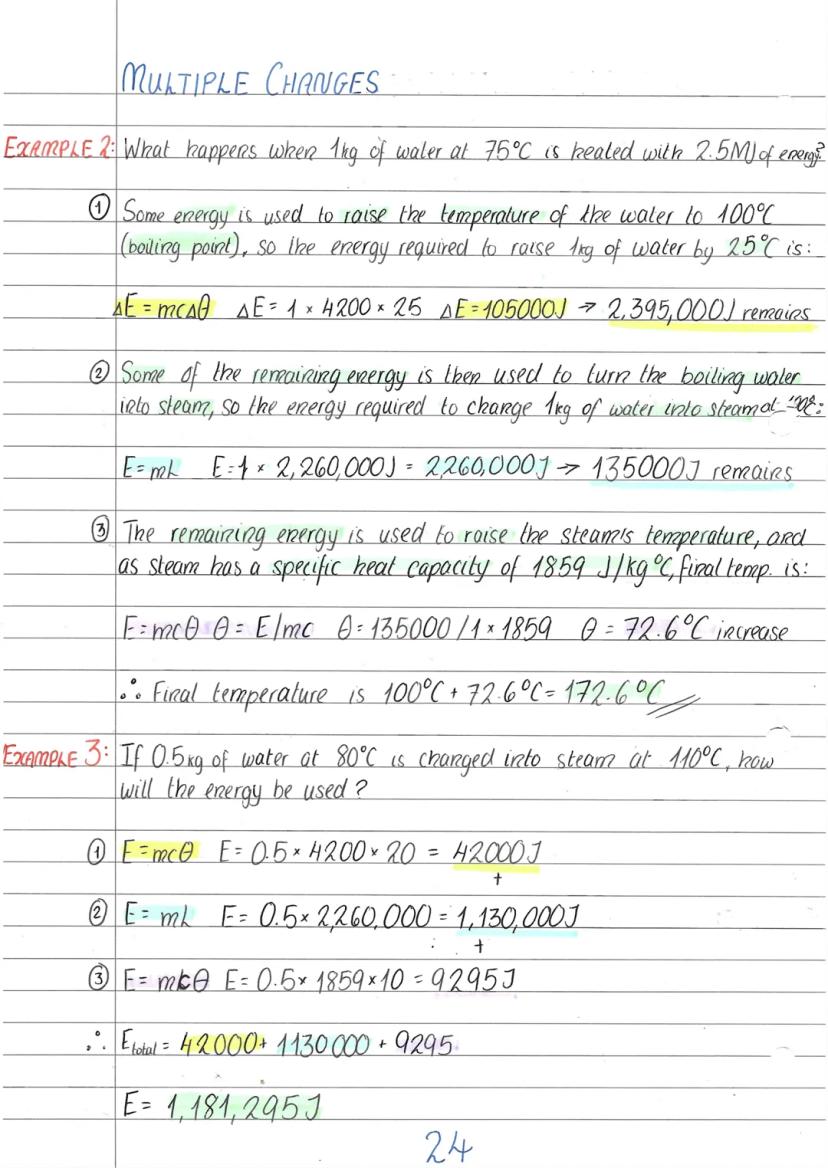
Real-world heating often involves several energy changes happening one after another. Imagine heating ice-cold water until it becomes superheated steam - you'll need energy for warming, melting, more warming, boiling, and even more warming!
The secret is breaking it down into stages. First, calculate the energy needed to reach the melting or boiling point using ΔE = mcΔθ. Then work out the energy for the state change using E = mL. Finally, calculate any further temperature changes in the new state.
Each state has its own specific heat capacity, so make sure you use the right value. Water is 4200 J/kg°C, but steam is only 1859 J/kg°C. This means steam heats up much faster than liquid water with the same energy input.
These calculations might look scary with all those big numbers, but just tackle them step by step. Write down what's happening at each stage, use the right formula, then add up all the energies at the end.
Calculator Tip: When dealing with millions of joules, convert to scientific notation early (like 2.5 × 10⁶) to avoid mistakes with all those zeros!

Density tells us how tightly packed the particles are in different materials - it's why a small piece of lead feels much heavier than a large piece of polystyrene. The formula ρ = m/V is one you'll use constantly.
Solids are usually densest because their particles are crammed together in regular patterns. Liquids are slightly less dense as particles can move around a bit more. Gases have really low density because particles are spread out over huge distances.
Different materials have wildly different densities even in the same state. A cube of iron will be much heavier than an identical cube of aluminium because iron atoms are more closely packed together.
For regular shapes like cubes or spheres, you can calculate volume using geometry formulas. The units matter too - make sure you're consistent with kg/m³ or g/cm³ throughout your calculations.
Unit Conversion: Remember that 1 g/cm³ = 1000 kg/m³. Water's density of 1 g/cm³ becomes 1000 kg/m³!

There are three main methods for measuring density, depending on what type of object you're investigating. For regular solids like cubes or spheres, simply measure dimensions with a ruler (or vernier callipers for better accuracy) and calculate the volume mathematically.
Irregular objects need the displacement method - this is where you use a eureka can filled to the spout with water. When you carefully lower your object in, the displaced water flows into a measuring cylinder, giving you the object's volume directly.
For liquids like water, measure a known volume (like 50 cm³) in a measuring cylinder, then find its mass using a balance. Remember to subtract the mass of the empty cylinder first to get just the liquid's mass.
Your results should match known values reasonably well - steel is around 7800 kg/m³, and water is exactly 1000 kg/m³. If your answers are way off, check your measurements and calculations for errors.
Practical Tip: Lower irregular objects into the displacement can really slowly to avoid splashing - you'll get much more accurate volume measurements this way!

Thermal energy always flows from hot to cold through three different methods: conduction, convection, and radiation. Each one works in completely different situations and involves different mechanisms.
Conduction happens in solids when vibrating particles bump into their neighbours, passing on kinetic energy like a Mexican wave through the material. Metals are brilliant conductors because they have delocalised electrons that can zoom around transferring energy quickly.
Convection only works in fluids (liquids and gases) where particles can actually move around. Hot, less dense regions rise whilst cooler, denser regions sink, creating convection currents - this is how your radiators heat your room and why hot air balloons float.
Radiation is completely different - it's electromagnetic waves (mainly infrared) that can travel through empty space. This is how the Sun's energy reaches Earth and why you feel warmth from a fire even when you're not touching it. Dark, rough surfaces are much better at absorbing and emitting radiation than shiny ones.
Real-world Connection: Your home uses all three methods - conduction through radiator pipes, convection currents warming the air, and radiant heat from the radiator surfaces!
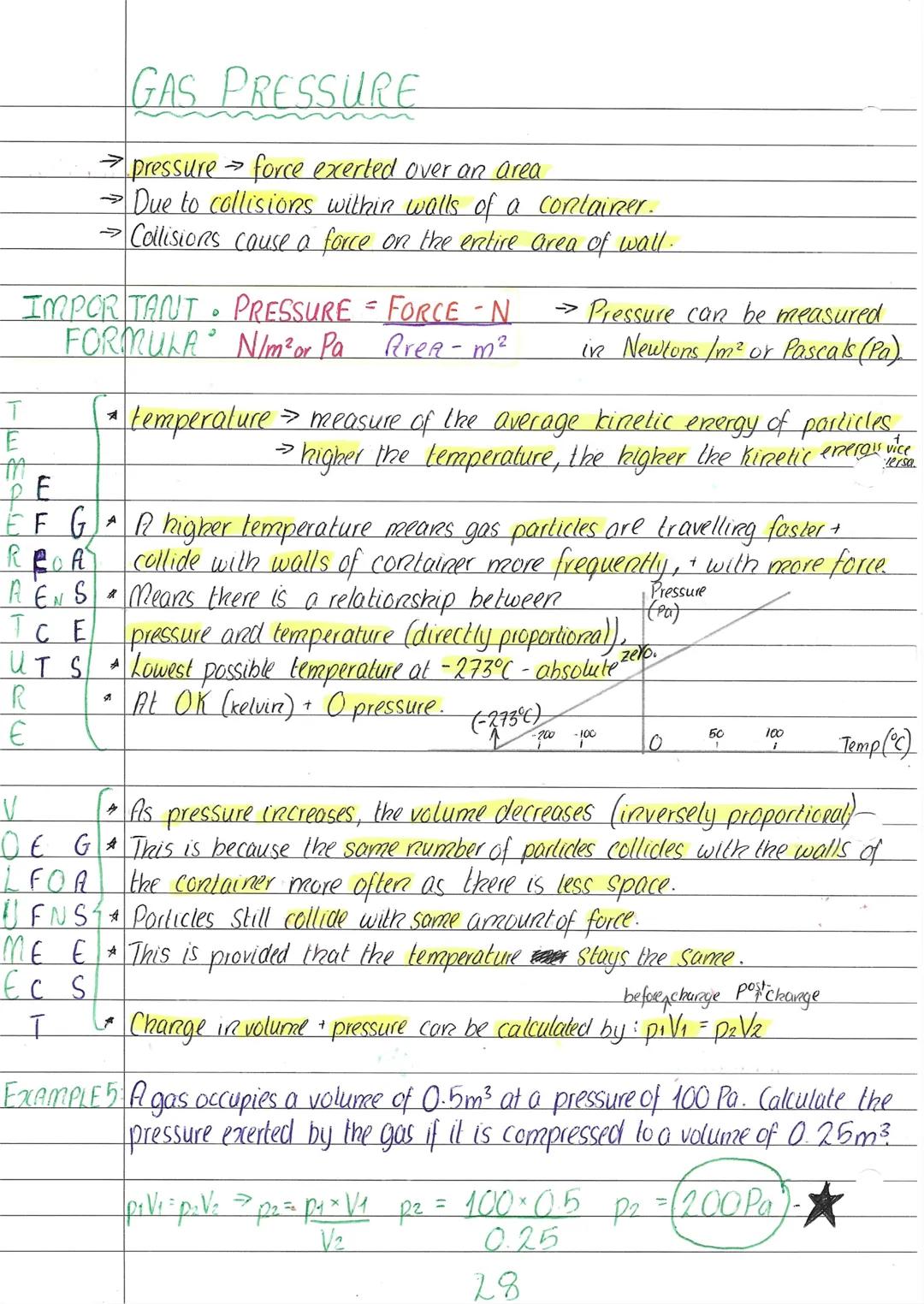
Gas pressure comes from billions of tiny particles constantly smashing into container walls - it's like being inside a room where invisible tennis balls are flying everywhere! The formula pressure = force ÷ area tells us that more collisions or harder collisions mean higher pressure.
Temperature is actually a measure of average particle kinetic energy. Higher temperature means faster-moving particles that hit walls more often and with greater force, so pressure and temperature are directly proportional.
Here's the clever bit: pressure and volume are inversely proportional (when temperature stays constant). Squash a gas into half the volume and the pressure doubles because particles hit the walls twice as often. The relationship p₁V₁ = p₂V₂ lets you calculate these changes.
The coldest possible temperature is absolute zero , where particles would theoretically stop moving completely and pressure would be zero. Obviously, this is impossible to reach in real life, but it's a useful concept for understanding gas behaviour.
Exam Strategy: Always check if temperature is constant when using p₁V₁ = p₂V₂. If temperature changes too, you'll need more complex gas law equations!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
TheOnionBhaji
@theonionbhaji7
Matter is made up of tiny particles that behave differently depending on whether they're in solids, liquids, or gases. Understanding how these particles move and interact helps explain everything from why ice melts to how your radiators heat your room.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why ice is hard but steam floats away? It's all about how particles are arranged and how much energy they have. In solids, particles are packed tightly in fixed positions, only vibrating on the spot like people standing shoulder-to-shoulder in a crowded lift.
Liquids have particles that are still close together but can slide around each other - imagine a busy dance floor where people move but stay relatively close. The particles have more energy than in solids, so the intermolecular forces holding them together are weaker.
Gases are completely different - particles zoom around randomly with loads of space between them, like footballs being kicked around an enormous pitch. They have high internal energy and very weak forces between particles. When you heat or cool materials, you can make them change state through processes like melting, boiling, freezing, and even sublimation (solid straight to gas).
Quick Tip: Remember that temperature stays constant during state changes - all the energy goes into breaking or forming bonds between particles!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Different materials need different amounts of energy to heat up - that's why a metal spoon gets scorching hot in your tea whilst a wooden one stays cool. Specific heat capacity tells us exactly how much energy we need to raise 1kg of a material by 1°C.
The key formula you'll need is ΔE = mcΔθ, where ΔE is the change in thermal energy, m is mass, c is specific heat capacity, and Δθ is the temperature change. Water has a particularly high specific heat capacity , which is why it takes ages to boil a kettle!
In the required practical, you'll heat a metal block with an electric heater whilst measuring the current, voltage, and temperature change. The tricky bit is that the block keeps heating up even after you switch off the heater, so you need to record the highest temperature it reaches.
Safety is crucial here - those heaters get dangerously hot, so never touch them directly. Always wrap your block in cotton wool for insulation to get accurate results.
Exam Tip: Energy from the heater equals current × time × voltage . Use this to find the total energy, then rearrange the main formula to find specific heat capacity!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's something mind-blowing: when ice melts or water boils, the temperature stays exactly the same even though you're still adding energy. That energy isn't disappearing - it's being used to break the bonds between particles instead of making them move faster.
Specific latent heat is the energy needed to change 1kg of a material from one state to another without changing temperature. Every material has two types: latent heat of fusion and latent heat of vaporisation .
The formula is simpler than specific heat capacity: E = mL, where L is the specific latent heat. You can spot these energy changes on heating curves - they're the flat horizontal bits where temperature stays constant whilst the material changes state.
For water, the latent heat of vaporisation is absolutely massive compared to fusion . This explains why steam burns are so much worse than boiling water - that steam releases enormous amounts of energy when it condenses on your skin.
Memory Trick: Think "L for state Lines" - latent heat happens along the horizontal lines on heating/cooling curves!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Real-world heating often involves several energy changes happening one after another. Imagine heating ice-cold water until it becomes superheated steam - you'll need energy for warming, melting, more warming, boiling, and even more warming!
The secret is breaking it down into stages. First, calculate the energy needed to reach the melting or boiling point using ΔE = mcΔθ. Then work out the energy for the state change using E = mL. Finally, calculate any further temperature changes in the new state.
Each state has its own specific heat capacity, so make sure you use the right value. Water is 4200 J/kg°C, but steam is only 1859 J/kg°C. This means steam heats up much faster than liquid water with the same energy input.
These calculations might look scary with all those big numbers, but just tackle them step by step. Write down what's happening at each stage, use the right formula, then add up all the energies at the end.
Calculator Tip: When dealing with millions of joules, convert to scientific notation early (like 2.5 × 10⁶) to avoid mistakes with all those zeros!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Density tells us how tightly packed the particles are in different materials - it's why a small piece of lead feels much heavier than a large piece of polystyrene. The formula ρ = m/V is one you'll use constantly.
Solids are usually densest because their particles are crammed together in regular patterns. Liquids are slightly less dense as particles can move around a bit more. Gases have really low density because particles are spread out over huge distances.
Different materials have wildly different densities even in the same state. A cube of iron will be much heavier than an identical cube of aluminium because iron atoms are more closely packed together.
For regular shapes like cubes or spheres, you can calculate volume using geometry formulas. The units matter too - make sure you're consistent with kg/m³ or g/cm³ throughout your calculations.
Unit Conversion: Remember that 1 g/cm³ = 1000 kg/m³. Water's density of 1 g/cm³ becomes 1000 kg/m³!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
There are three main methods for measuring density, depending on what type of object you're investigating. For regular solids like cubes or spheres, simply measure dimensions with a ruler (or vernier callipers for better accuracy) and calculate the volume mathematically.
Irregular objects need the displacement method - this is where you use a eureka can filled to the spout with water. When you carefully lower your object in, the displaced water flows into a measuring cylinder, giving you the object's volume directly.
For liquids like water, measure a known volume (like 50 cm³) in a measuring cylinder, then find its mass using a balance. Remember to subtract the mass of the empty cylinder first to get just the liquid's mass.
Your results should match known values reasonably well - steel is around 7800 kg/m³, and water is exactly 1000 kg/m³. If your answers are way off, check your measurements and calculations for errors.
Practical Tip: Lower irregular objects into the displacement can really slowly to avoid splashing - you'll get much more accurate volume measurements this way!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Thermal energy always flows from hot to cold through three different methods: conduction, convection, and radiation. Each one works in completely different situations and involves different mechanisms.
Conduction happens in solids when vibrating particles bump into their neighbours, passing on kinetic energy like a Mexican wave through the material. Metals are brilliant conductors because they have delocalised electrons that can zoom around transferring energy quickly.
Convection only works in fluids (liquids and gases) where particles can actually move around. Hot, less dense regions rise whilst cooler, denser regions sink, creating convection currents - this is how your radiators heat your room and why hot air balloons float.
Radiation is completely different - it's electromagnetic waves (mainly infrared) that can travel through empty space. This is how the Sun's energy reaches Earth and why you feel warmth from a fire even when you're not touching it. Dark, rough surfaces are much better at absorbing and emitting radiation than shiny ones.
Real-world Connection: Your home uses all three methods - conduction through radiator pipes, convection currents warming the air, and radiant heat from the radiator surfaces!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Gas pressure comes from billions of tiny particles constantly smashing into container walls - it's like being inside a room where invisible tennis balls are flying everywhere! The formula pressure = force ÷ area tells us that more collisions or harder collisions mean higher pressure.
Temperature is actually a measure of average particle kinetic energy. Higher temperature means faster-moving particles that hit walls more often and with greater force, so pressure and temperature are directly proportional.
Here's the clever bit: pressure and volume are inversely proportional (when temperature stays constant). Squash a gas into half the volume and the pressure doubles because particles hit the walls twice as often. The relationship p₁V₁ = p₂V₂ lets you calculate these changes.
The coldest possible temperature is absolute zero , where particles would theoretically stop moving completely and pressure would be zero. Obviously, this is impossible to reach in real life, but it's a useful concept for understanding gas behaviour.
Exam Strategy: Always check if temperature is constant when using p₁V₁ = p₂V₂. If temperature changes too, you'll need more complex gas law equations!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
29
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the principles of conduction and convection in liquids and gases. This summary covers thermal conductivity, energy transfer, and the behavior of particles during heating and cooling. Ideal for GCSE Physics students seeking to understand how heat moves through different states of matter.
Explore the evolution of atomic theory, from early concepts by Democritus to modern atomic models. Understand key principles such as atomic structure, periodic table development, and the role of isotopes. This summary covers essential topics including energy forms, oxidation states, and the contributions of notable scientists like Dalton, Thompson, and Bohr. Ideal for students studying chemistry and physics.
Explore the fundamentals of atomic structure, including key concepts such as nuclear fission, fusion, radioactive decay, and the properties of subatomic particles. This comprehensive resource includes past exam questions and mark schemes to help you identify and strengthen your understanding of atomic theory and its applications in physics.
Explore the fundamentals of nuclear decay, atomic structure, and radiation types in this comprehensive summary. Key concepts include activity measurement, half-life, radioactive decay, fission, fusion, and the historical development of atomic models. Ideal for AQA students preparing for exams.
Explore the fundamentals of atomic structure and nuclear radiation in this comprehensive study resource. Covering key concepts such as isotopes, radioactive decay, nuclear fission, and the historical development of atomic models, this summary is designed for GCSE Physics students preparing for AQA Higher Triple exams. Enhance your understanding of protons, neutrons, and the properties of alpha, beta, and gamma radiation.
Explore the fundamentals of radioactivity, including alpha, beta, and gamma decay, half-life calculations, and the properties of isotopes. This summary covers key concepts such as nuclear radiation, energy levels, and the differences between contamination and irradiation. Ideal for students preparing for exams in combined science.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user