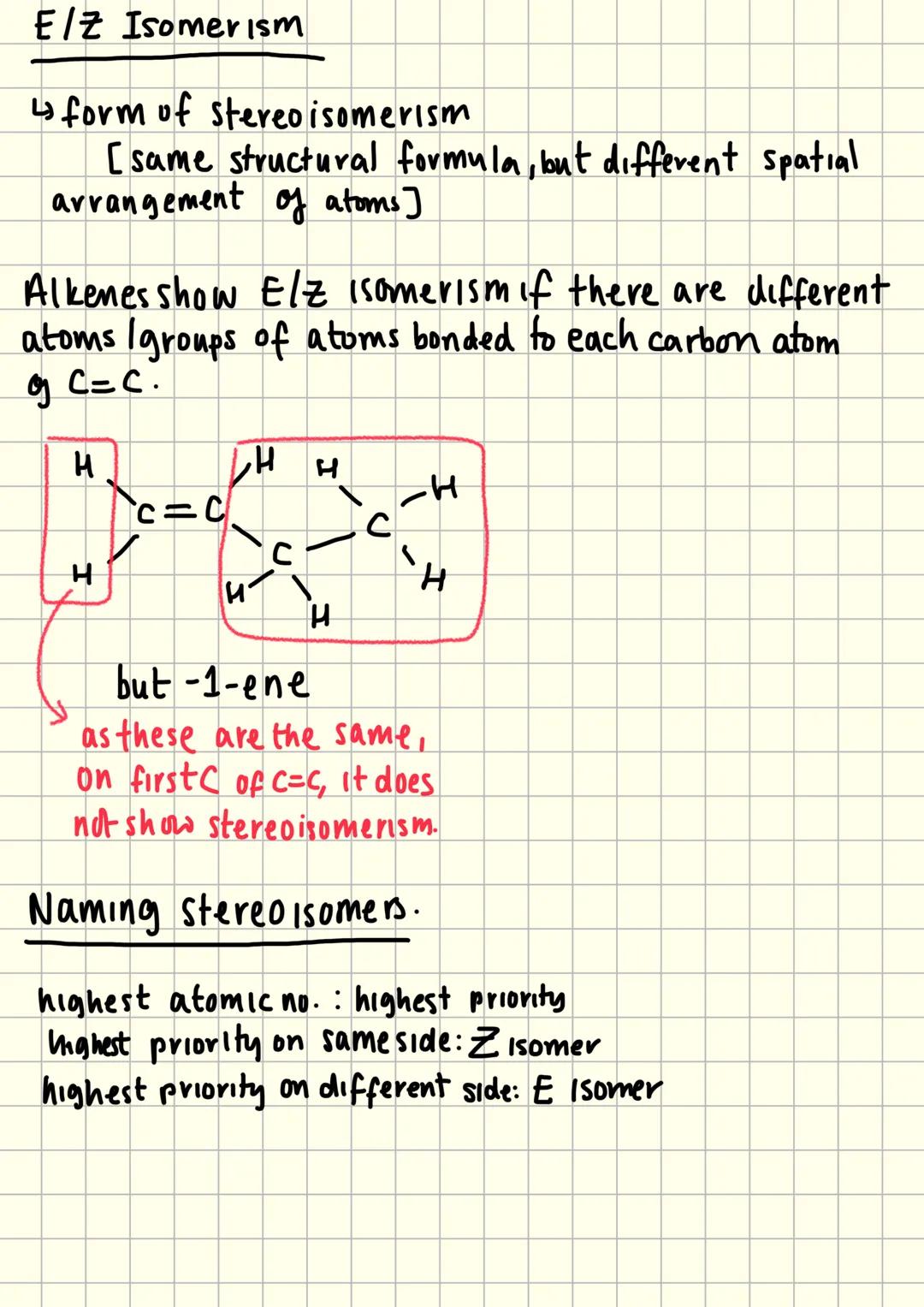
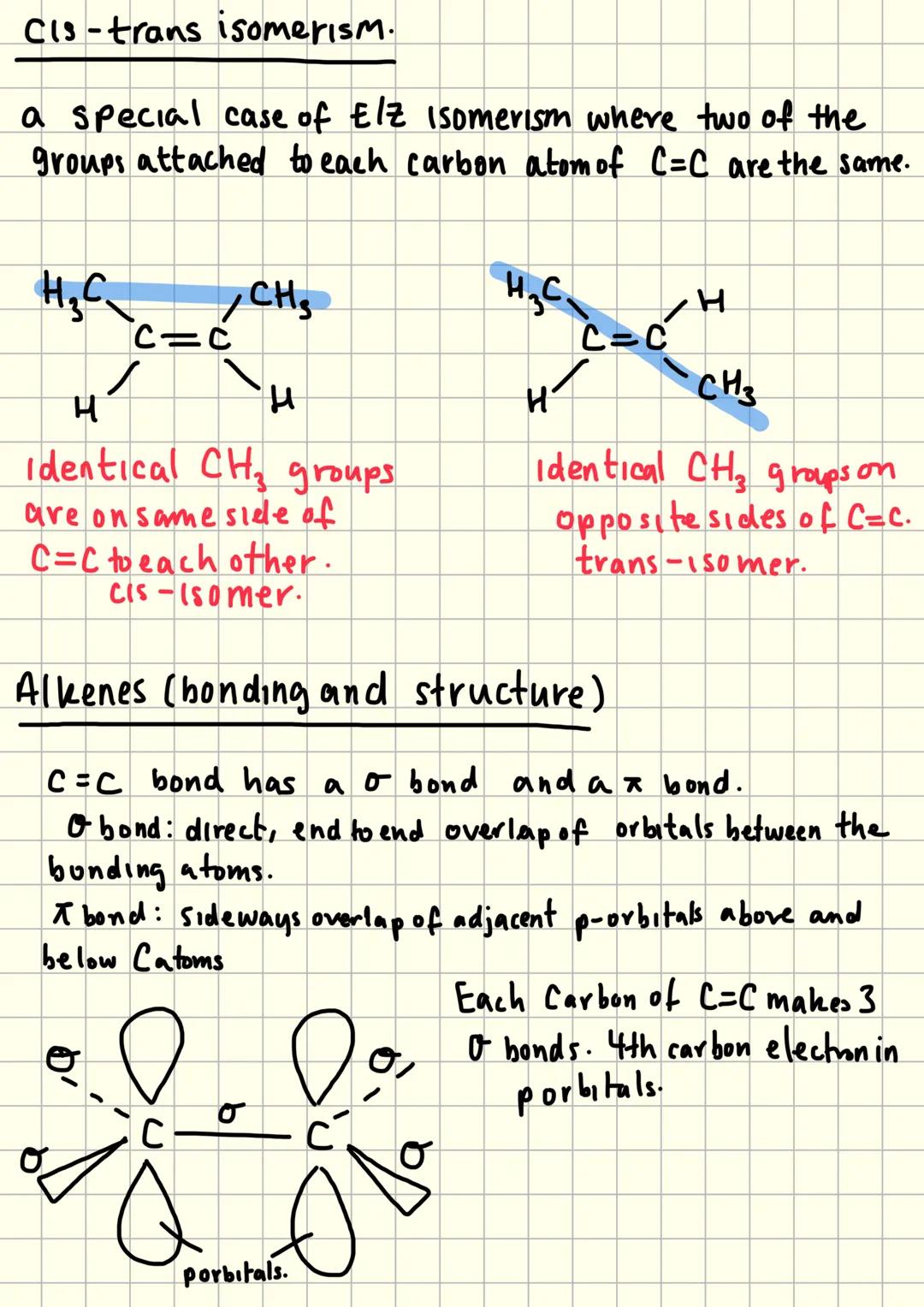
Radical Substitution with Halogens
When alkanes meet halogens under UV light, things get interesting through radical substitution. This three-stage mechanism involves highly reactive radicals - atoms or molecules with unpaired electrons.
Initiation starts when UV light breaks the halogen molecule by homolytic fission, creating two radical atoms. Propagation involves chain reactions where radicals react to form products whilst generating new radicals to continue the process.
Termination occurs when two radicals combine to form stable molecules, ending the chain reaction. The problem with this reaction is that substitution can happen anywhere on the carbon chain, creating multiple products.
For your exams, you need to write mechanisms clearly. Start with initiation (showing homolytic fission with UV), then show two propagation steps, and finish with possible termination reactions.
Memory Trick: Think of radical substitution as a chain reaction - once it starts, it keeps going until radicals run out!