Understanding Electrolysis
Ever wondered how we extract pure metals from compounds? Electrolysis uses an electric current to decompose molten or dissolved ionic compounds. Think of it as using electricity to "crack open" compounds and separate their parts.
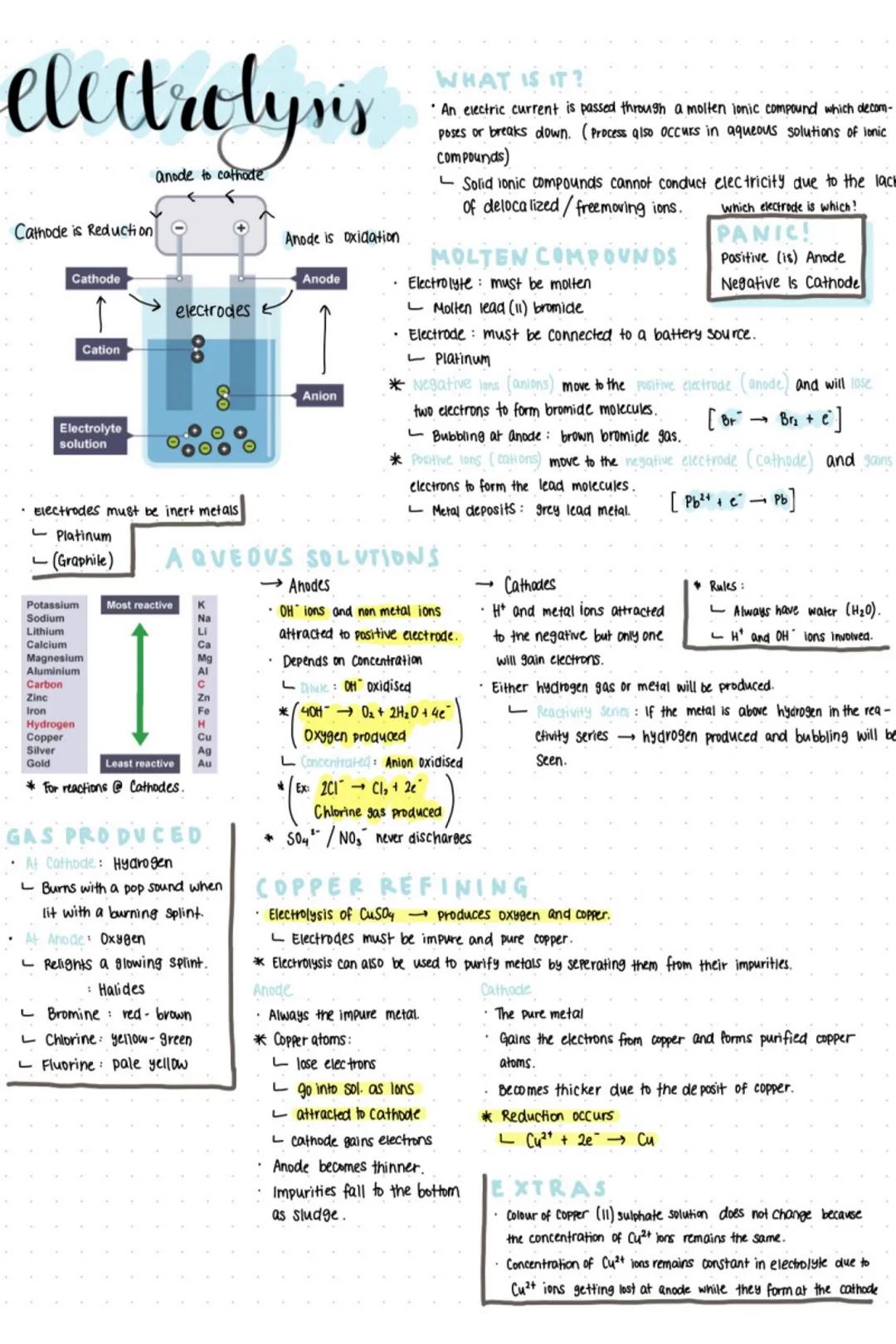
The setup requires three key components: electrodes (usually made of inert metals like platinum), an electrolyte (the ionic compound being broken down), and a power source. Remember the handy acronym PANIC - Positive is Anode, Negative Is Cathode. The anode is where oxidation occurs (electrons are lost), whilst the cathode is where reduction happens (electrons are gained).
In molten compounds like lead bromide, positive ions (cations) move towards the negative cathode to gain electrons, forming pure metal. Meanwhile, negative ions (anions) head to the positive anode, losing electrons to form gases. You'll see grey lead metal depositing at the cathode and brown bromine gas bubbling at the anode.
Key Tip: Solid ionic compounds can't conduct electricity because their ions aren't free to move - that's why we need to melt them first!
Aqueous solutions are trickier because water creates H⁺ and OH⁻ ions that compete with your original compound. The reactivity series determines what actually gets produced - if your metal is more reactive than hydrogen, you'll get hydrogen gas bubbling at the cathode instead of metal deposits.