Chemistry isn't as scary as it seems - it's basically... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
243
•
7 Feb 2026
•
Kaif Hossain
@aifossain_cbyttpcfhr
Chemistry isn't as scary as it seems - it's basically... Show more




Ever wondered what makes gold different from iron? It's all down to elements - there are 118 different types that scientists have discovered, each with its own special chemical symbol like a secret code. Atoms are the tiniest possible pieces of these elements, like the ultimate LEGO blocks of the universe.
When atoms from different elements get together, they create compounds. Think of it like making a recipe - combine hydrogen and oxygen atoms, and you get water! These compounds have formulae that show exactly which elements are mixed together.
Chemical reactions are where the magic happens. They're like atomic dance parties where compounds break apart or form new partnerships, creating at least one completely new substance with a measurable energy change.
Key tip: In any chemical equation, reactants (the starting materials) go on the left, and products (what you end up with) go on the right!

Here's something brilliant about chemistry - atoms never disappear or pop into existence during reactions. That's why symbol equations must be balanced, with the same number of each type of atom on both sides. It's like a perfectly fair trade!
Mixtures are completely different from compounds - they're just substances hanging out together without any chemical bonding. You can separate them easily because they keep their individual properties. It's like having a bowl of mixed sweets - they're all together, but each sweet is still exactly what it was before.
Homogeneous mixtures are so well blended you can't see the separate parts (like dissolved sugar in tea). Heterogeneous mixtures are the opposite - you can spot the different bits easily (like a fruit salad).
Purity check: Pure substances have exact melting and boiling points. Pure water always boils at 100°C - if it boils higher, there's something dissolved in it!

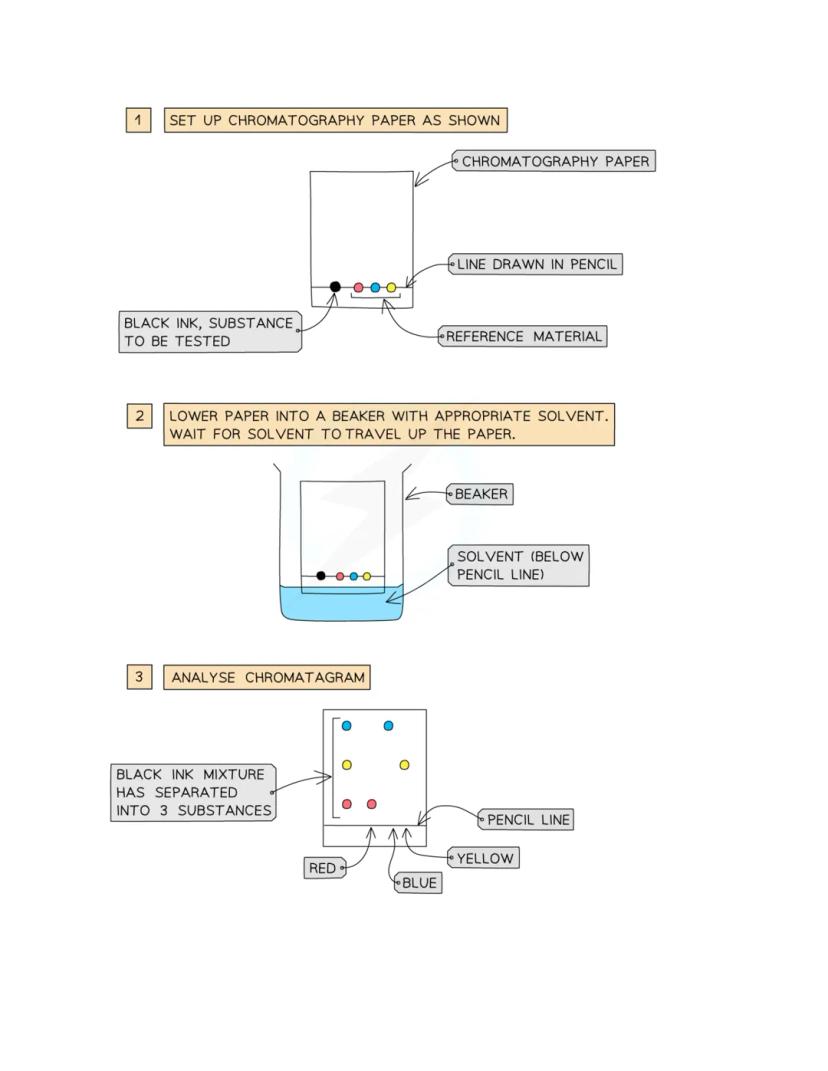
Chromatography is like giving different substances a race up a piece of paper! It's perfect for separating mixtures with different solubilities - think of splitting black ink back into its original colours.
The setup is dead simple: draw a pencil line on chromatography paper , spot your sample on it, then dip the paper into solvent. Make sure that pencil line stays above the solvent level, or your sample will wash away.
As the solvent travels up by capillary action, it carries the different substances at different speeds. The more soluble a substance is, the further it travels. This creates a beautiful pattern showing all the hidden components in your original mixture.
Pro tip: Always use pencil for the starting line because ink would interfere with your results by running up the paper too!

The chromatography setup follows three simple steps that even work brilliantly at home! First, you prepare your chromatography paper with a pencil line and carefully place dots of your sample (like black ink) on it.
Next comes the exciting bit - you lower the paper into a beaker with the right solvent, making sure the pencil line sits above the liquid. Then you just wait and watch as the solvent climbs up the paper, carrying different coloured substances at different rates.
Finally, you get to analyse your chromatogram! If you started with black ink, you might discover it's actually made from yellow, red, and blue components that have now separated into distinct bands.
Amazing fact: This technique can reveal the secret ingredients in everything from food colourings to crime scene evidence!
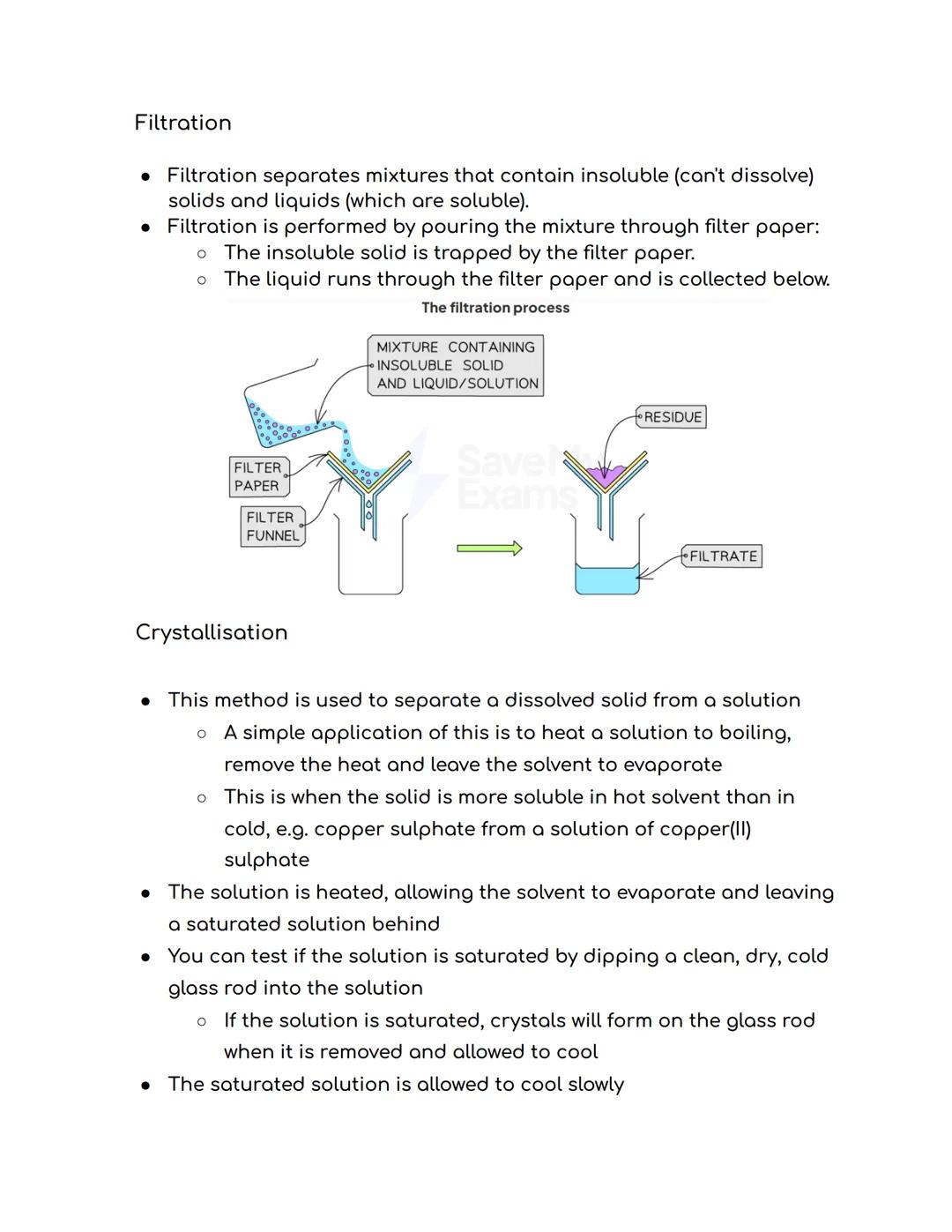
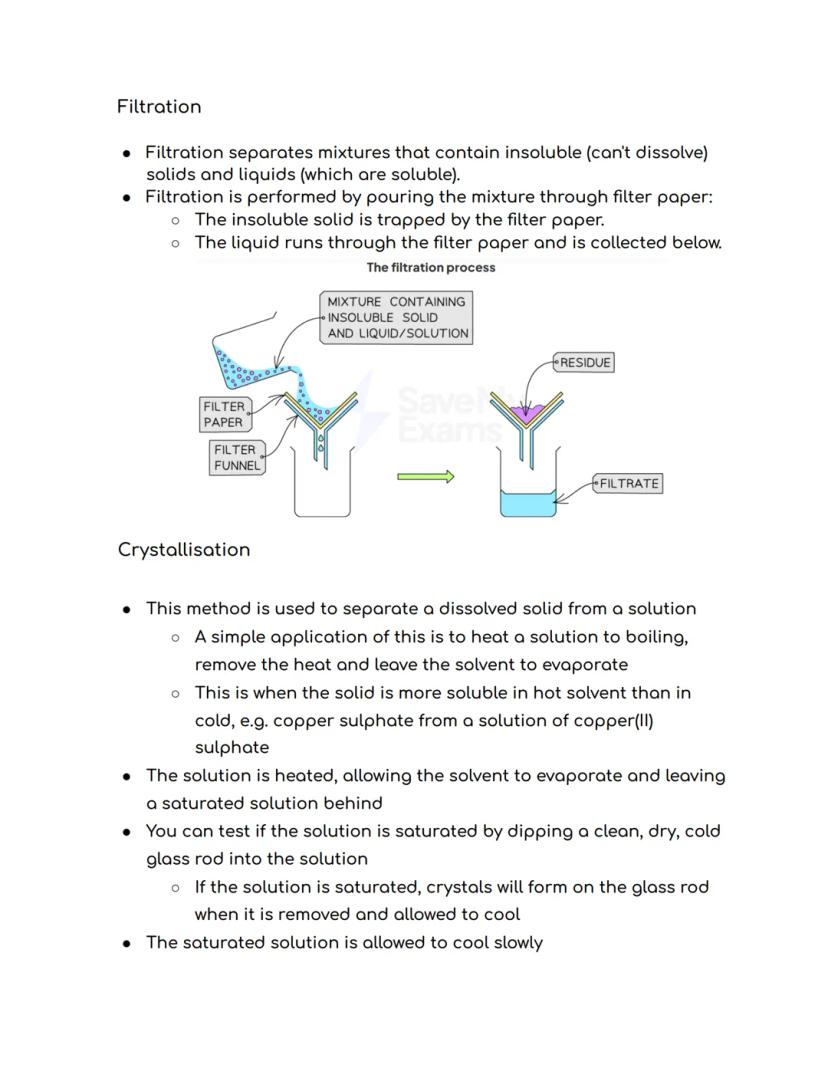
Filtration is your go-to method when you need to separate insoluble solids from liquids. Picture making coffee - the filter paper traps the coffee grounds whilst letting the liquid through. The trapped solid is called the residue, and the liquid that passes through is the filtrate.
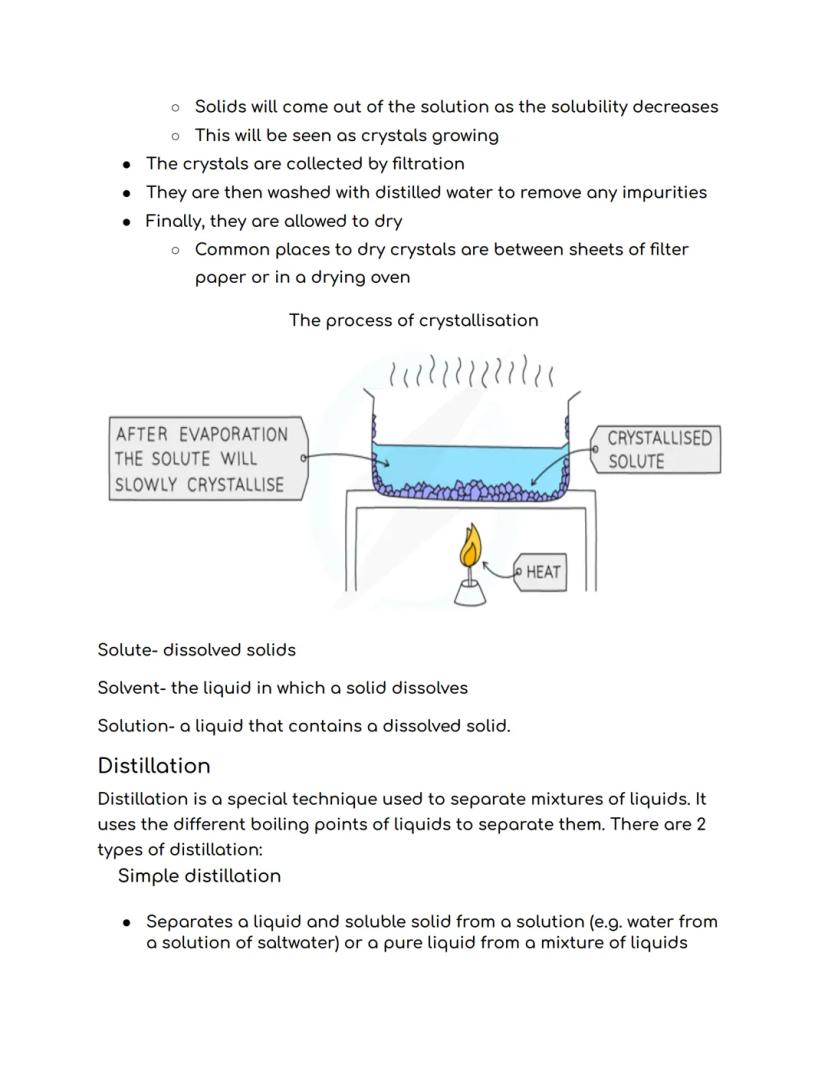
Crystallisation works like magic to separate dissolved solids from solutions. You heat the solution to make water evaporate, leaving behind a saturated solution - basically, liquid that's holding as much dissolved solid as physically possible.
Here's a cool test: dip a clean, dry, cold glass rod into your heated solution. If crystals form on it when you pull it out, you've got a saturated solution! Let it cool slowly, and beautiful crystals will grow as the solid becomes less soluble in the cooler liquid.
Crystal care: Always wash your final crystals with distilled water and dry them properly between filter papers or in a drying oven.
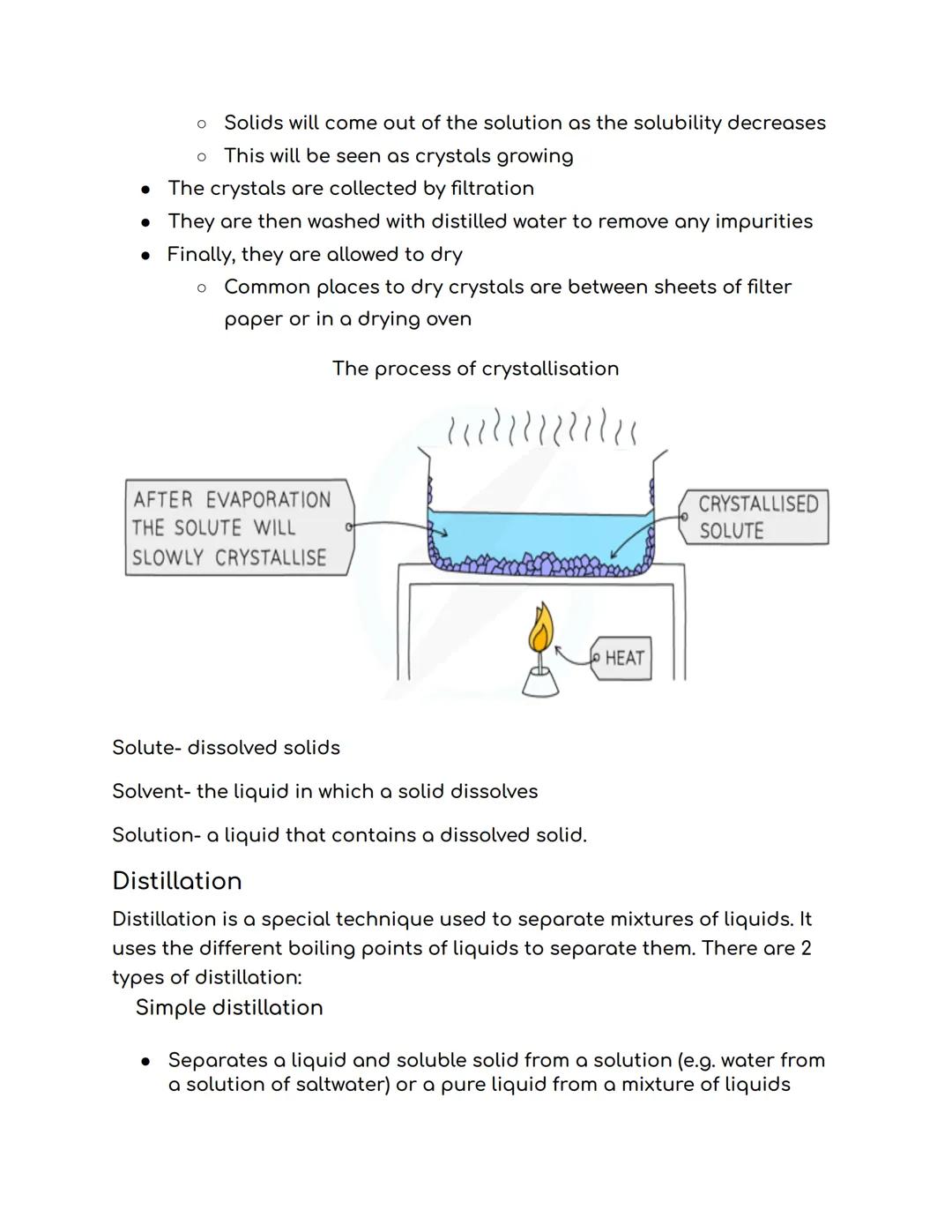
Before diving into distillation, get these terms sorted: the solute is your dissolved solid, the solvent is the liquid doing the dissolving, and together they make a solution. Think tea again - sugar is the solute, water is the solvent, and sweet tea is your solution!
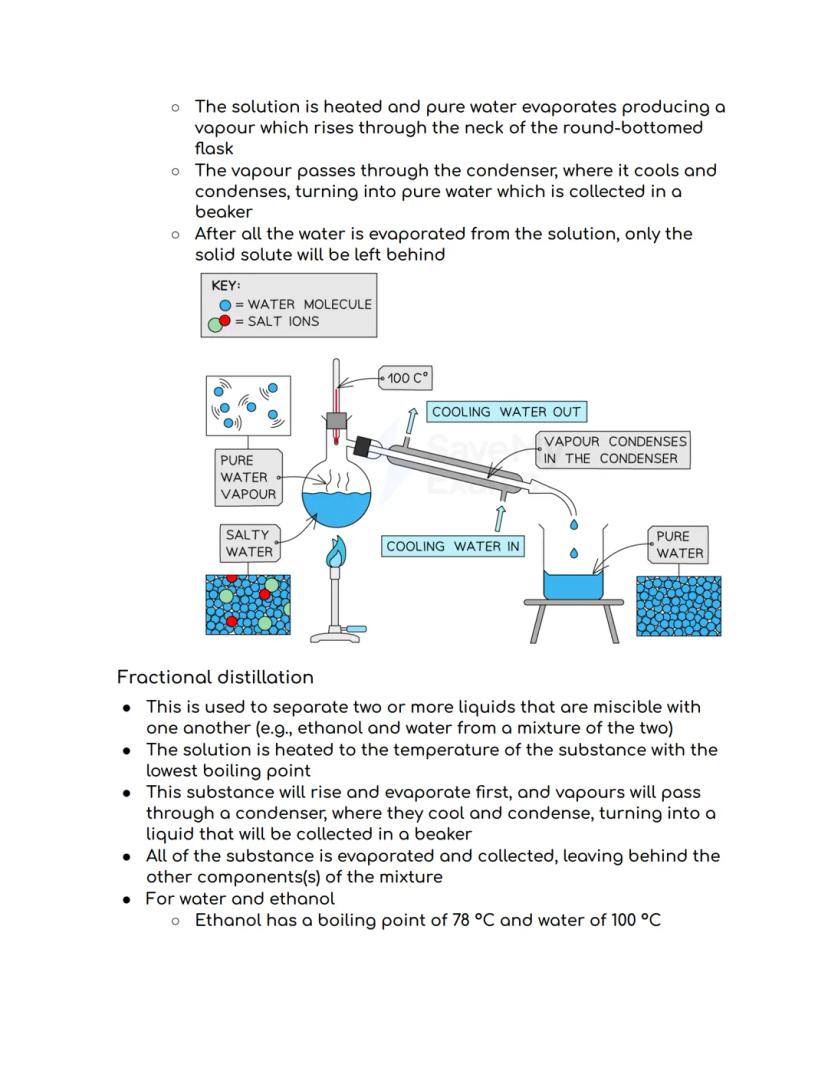
Simple distillation is brilliant for separating liquids from dissolved solids, like getting pure water from salty seawater. You heat the solution until the water evaporates and rises as vapour, then cool it back down in a condenser to collect pure liquid.
The process is beautifully simple - vapour travels through the condenser where cooling water flowing around it turns the vapour back into pure liquid. Meanwhile, all the dissolved salt gets left behind in the original container.
Temperature matters: Different substances have different boiling points, which is exactly what makes distillation work so well!
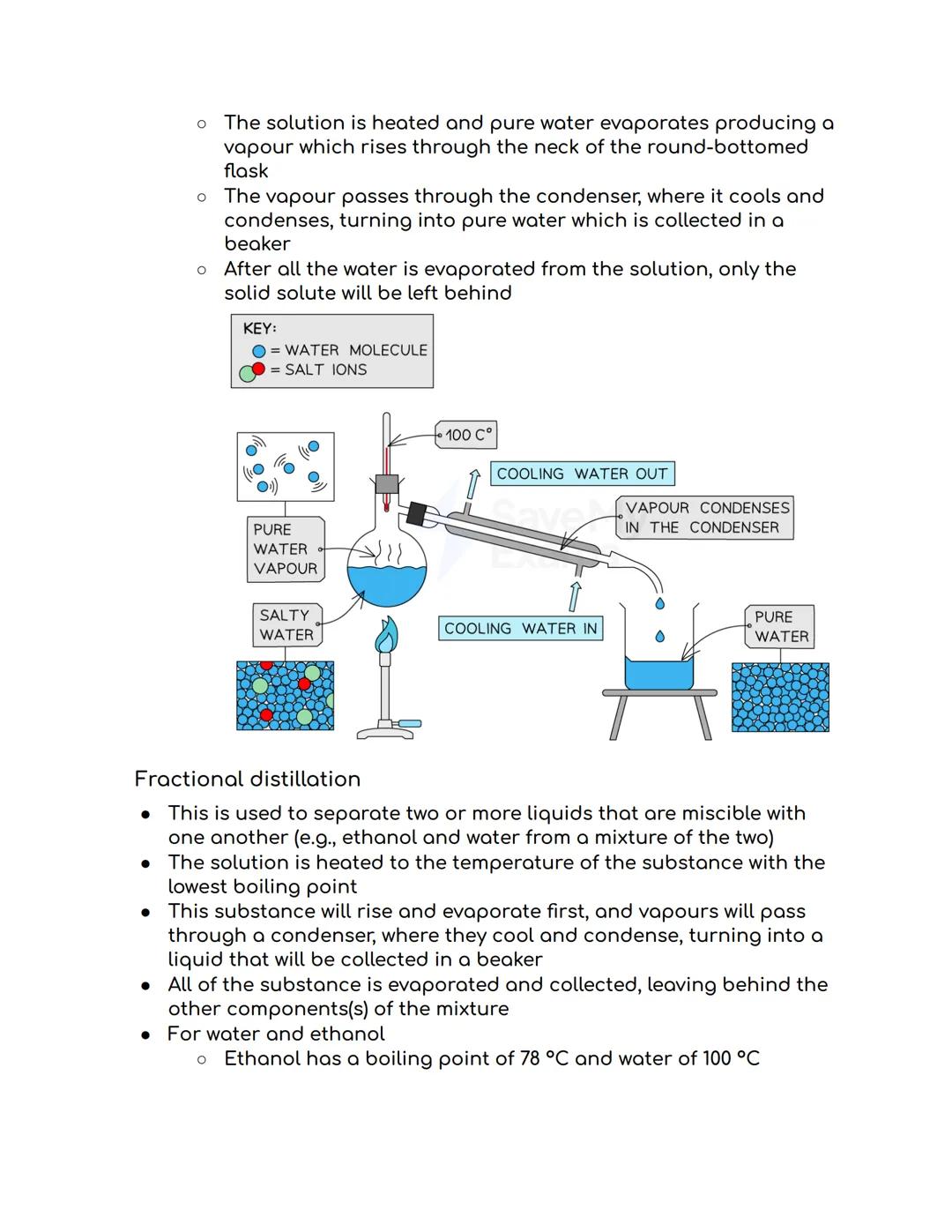
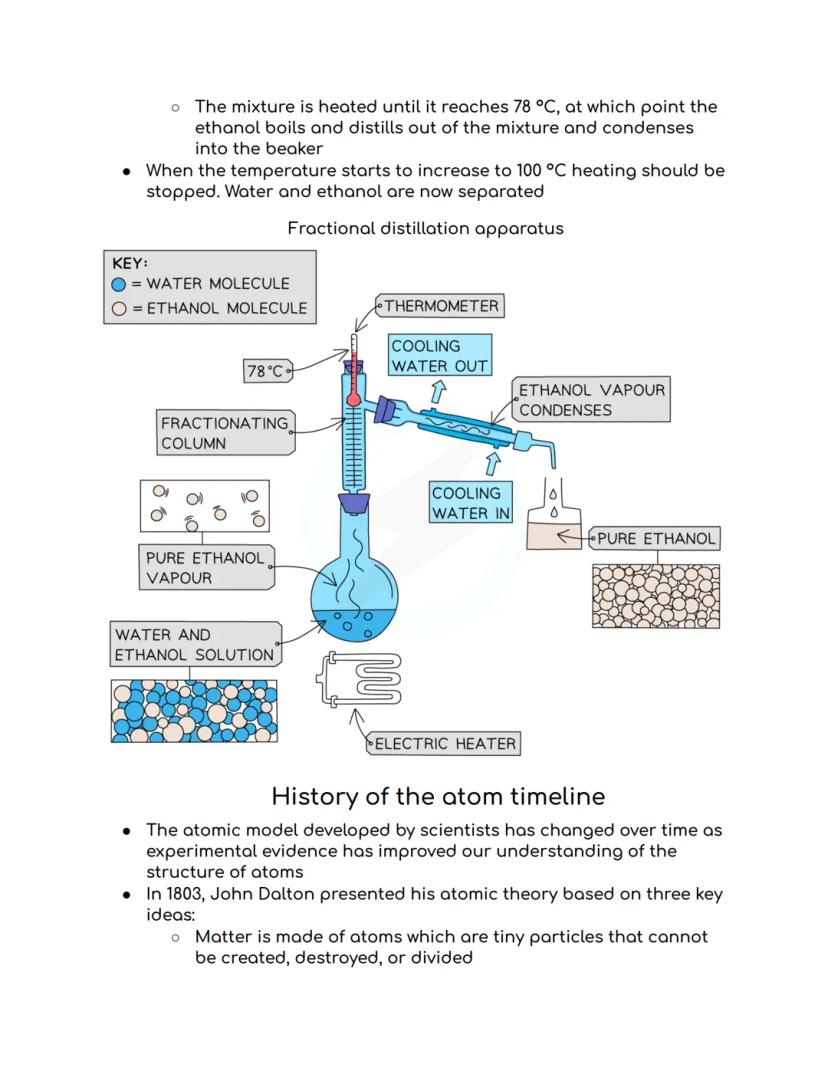
Fractional distillation is like simple distillation's clever cousin - it separates two or more liquids that mix together completely (called miscible liquids). Think separating alcohol from water, which is exactly how spirits are made!
The trick is using different boiling points. You heat the mixture to the temperature of whichever liquid boils first. For ethanol and water, ethanol boils at 78°C whilst water needs 100°C, so the ethanol evaporates first and gets collected separately.
The fractionating column makes this super efficient by providing extra surface area for separation. You stop heating when the temperature starts climbing towards the next liquid's boiling point - job done, perfectly separated liquids!
Real-world example: This is exactly how crude oil gets separated into petrol, diesel, and other useful products at refineries!
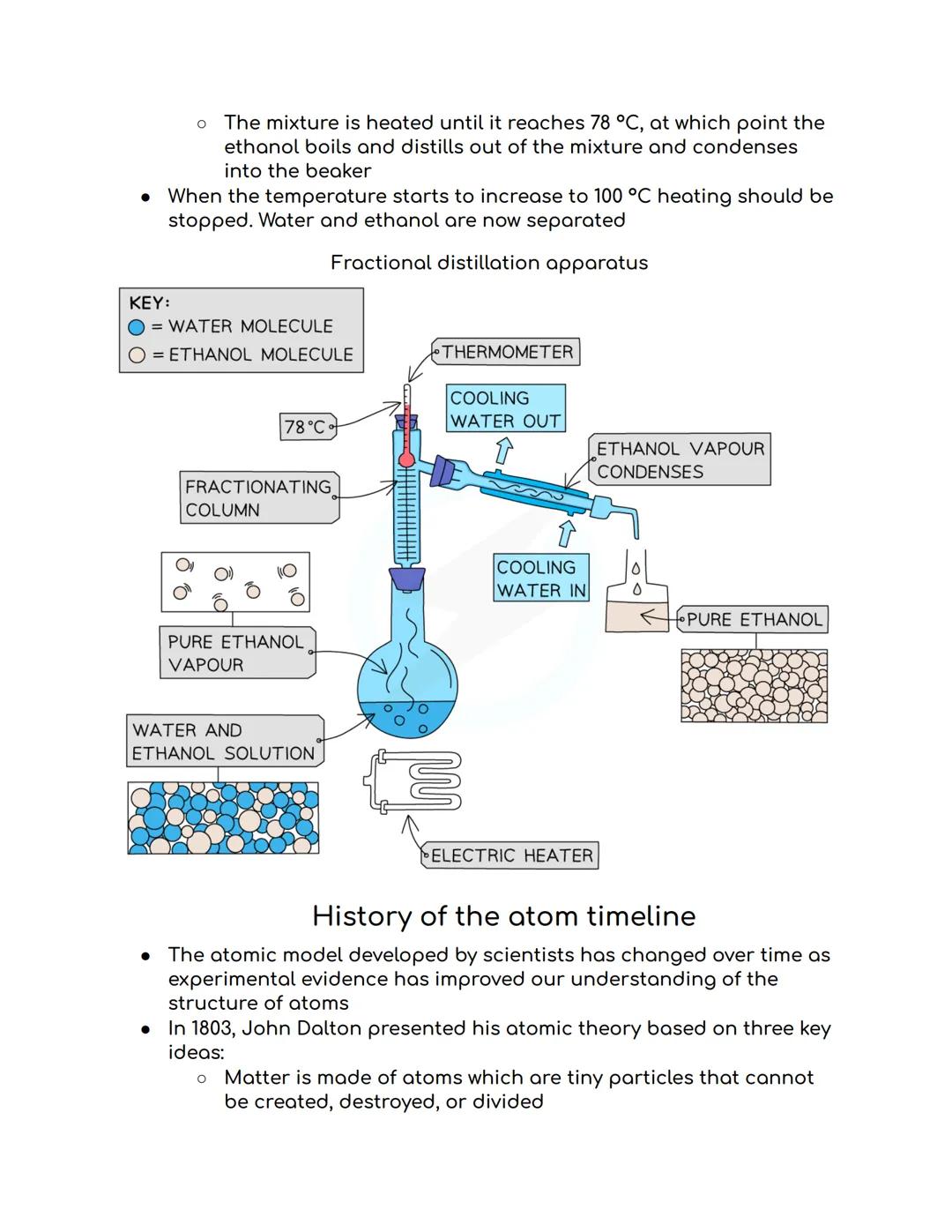
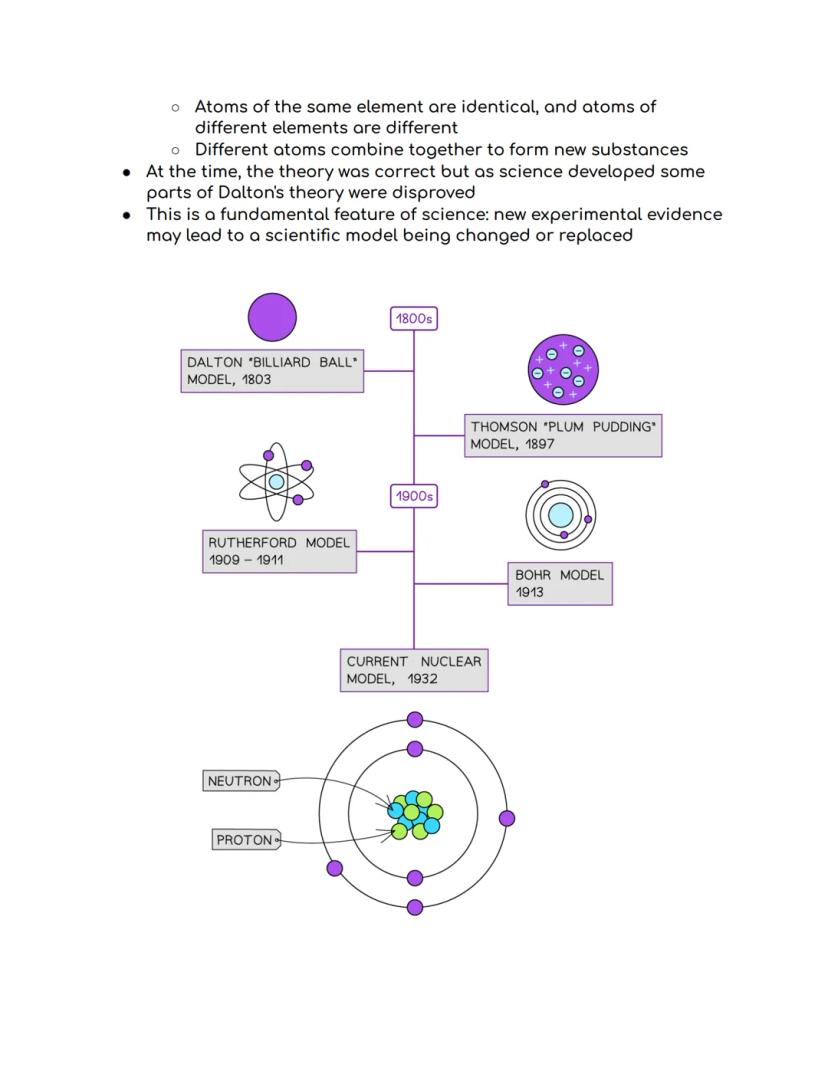
The story of atomic theory is like a detective story where each scientist built on previous discoveries! In 1803, John Dalton started it all with his atomic theory, suggesting matter was made of tiny, indestructible particles called atoms.
Dalton's key ideas were groundbreaking: atoms can't be created or destroyed, atoms of the same element are identical, and different atoms combine to make new substances. His "billiard ball" model pictured atoms as solid, indivisible spheres.
Fractional distillation continues with precise temperature control - when your ethanol and water mixture hits exactly 78°C, the ethanol boils off first. Stop heating when the temperature starts rising towards 100°C, and you've successfully separated them!
Science evolution: Dalton's theory was brilliant for its time, but science keeps improving as new evidence emerges!
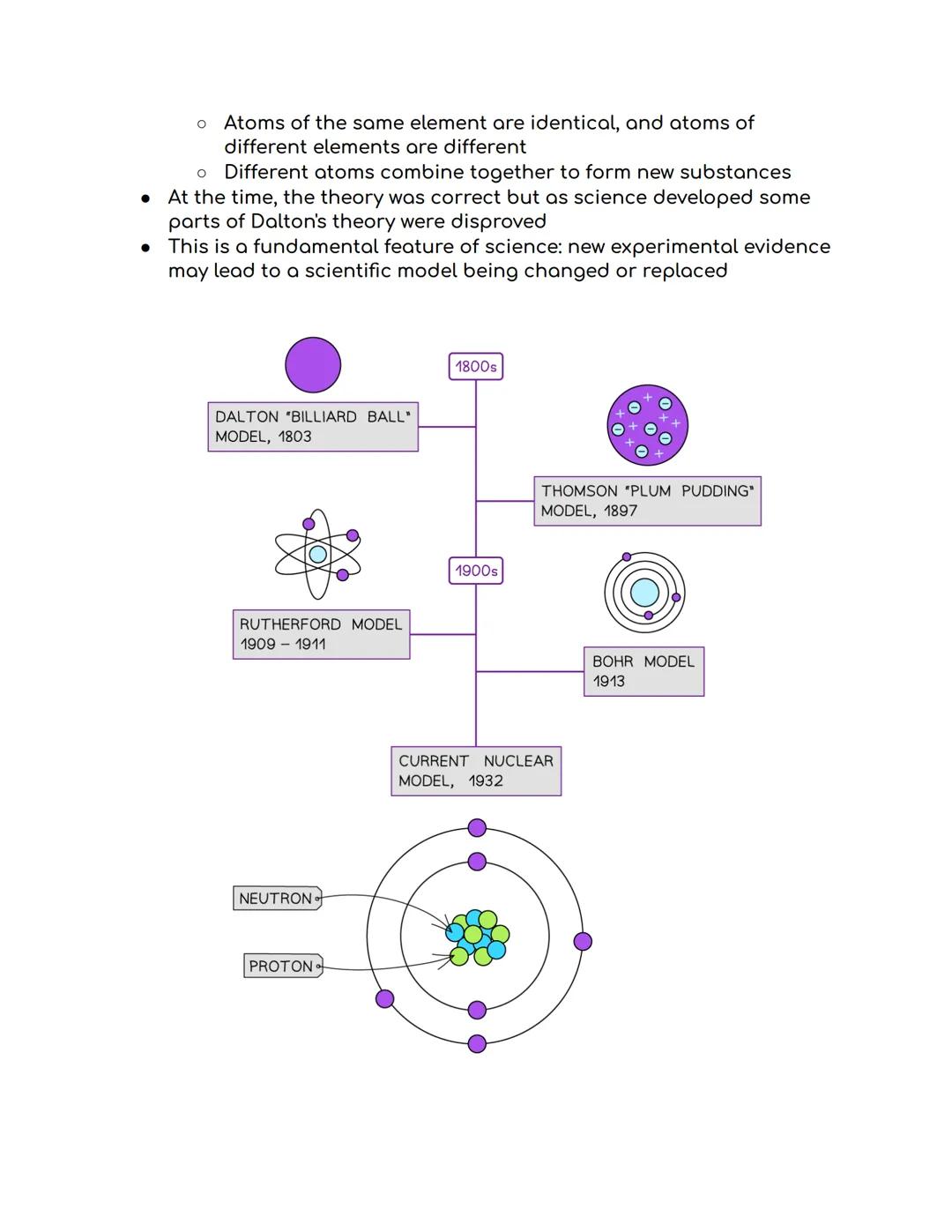
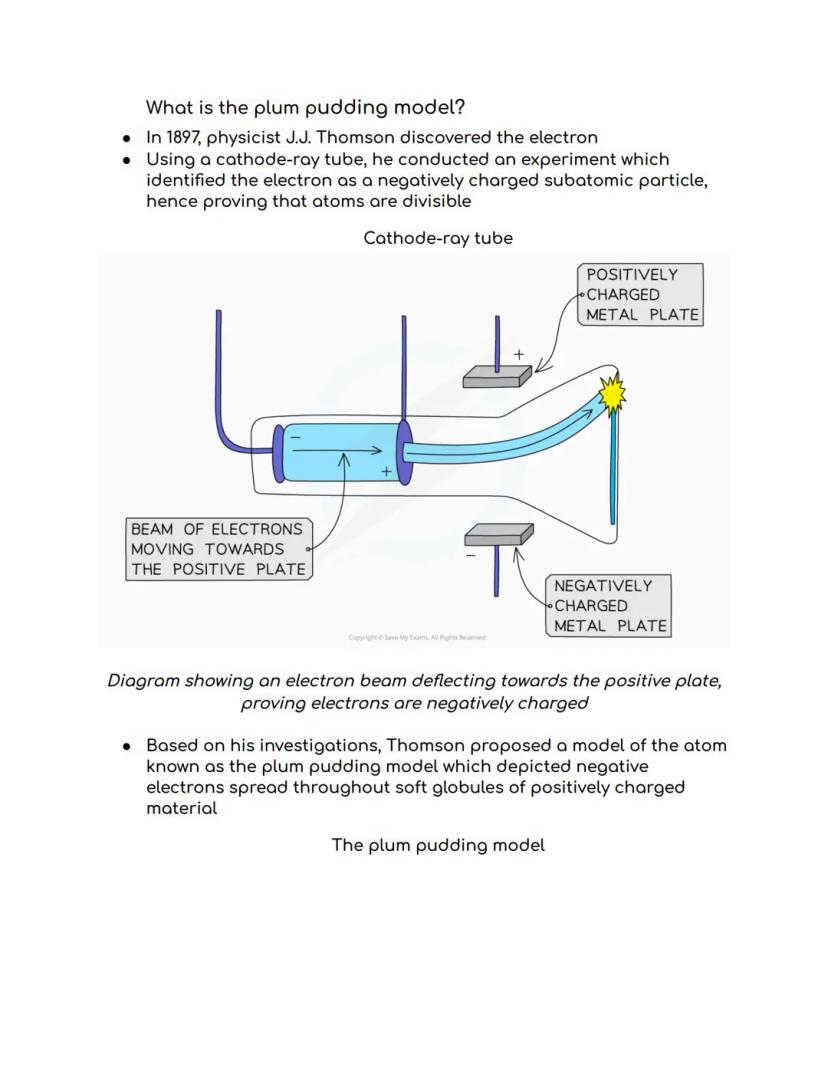
In 1897, J.J. Thomson completely revolutionised atomic theory by discovering the electron using a clever cathode-ray tube experiment. He showed that mysterious rays in the tube were actually streams of negatively charged particles - proving atoms weren't indivisible after all!
Thomson's experiment was ingenious: he created a beam of particles and watched them bend towards a positively charged plate. Since opposite charges attract, this proved the particles (electrons) were negatively charged. Dalton's "billiard ball" model was officially outdated!
This led to Thomson's "plum pudding" model - imagine a Christmas pudding where the pudding itself is positive and the raisins are negative electrons scattered throughout. It wasn't perfect, but it was a massive step forward in understanding atomic structure.
Game changer: Thomson's discovery proved that atoms have internal structure and aren't just solid balls!

Thomson's cathode-ray tube experiment was absolutely brilliant in its simplicity. He set up two metal plates - one positive, one negative - and watched as a mysterious beam curved towards the positive plate. This bending proved the beam was made of negatively charged particles.
The plum pudding model that emerged from this discovery pictured atoms as soft, positively charged material with electrons scattered throughout like raisins in a pudding. While we now know this isn't quite right, it was revolutionary for proving atoms had internal structure.
This discovery opened the floodgates for modern chemistry and physics. Thomson showed that atoms weren't the smallest particles after all - they contained even tinier subatomic particles that could be studied and understood.
Scientific method: Thomson's work perfectly shows how science progresses - each discovery builds on previous knowledge while sometimes overturning old ideas!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Kaif Hossain
@aifossain_cbyttpcfhr
Chemistry isn't as scary as it seems - it's basically about understanding how everything around you is built from tiny building blocks called atoms! You'll learn how these atoms combine to make everything from water to your mobile phone, plus... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered what makes gold different from iron? It's all down to elements - there are 118 different types that scientists have discovered, each with its own special chemical symbol like a secret code. Atoms are the tiniest possible pieces of these elements, like the ultimate LEGO blocks of the universe.
When atoms from different elements get together, they create compounds. Think of it like making a recipe - combine hydrogen and oxygen atoms, and you get water! These compounds have formulae that show exactly which elements are mixed together.
Chemical reactions are where the magic happens. They're like atomic dance parties where compounds break apart or form new partnerships, creating at least one completely new substance with a measurable energy change.
Key tip: In any chemical equation, reactants (the starting materials) go on the left, and products (what you end up with) go on the right!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's something brilliant about chemistry - atoms never disappear or pop into existence during reactions. That's why symbol equations must be balanced, with the same number of each type of atom on both sides. It's like a perfectly fair trade!
Mixtures are completely different from compounds - they're just substances hanging out together without any chemical bonding. You can separate them easily because they keep their individual properties. It's like having a bowl of mixed sweets - they're all together, but each sweet is still exactly what it was before.
Homogeneous mixtures are so well blended you can't see the separate parts (like dissolved sugar in tea). Heterogeneous mixtures are the opposite - you can spot the different bits easily (like a fruit salad).
Purity check: Pure substances have exact melting and boiling points. Pure water always boils at 100°C - if it boils higher, there's something dissolved in it!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chromatography is like giving different substances a race up a piece of paper! It's perfect for separating mixtures with different solubilities - think of splitting black ink back into its original colours.
The setup is dead simple: draw a pencil line on chromatography paper , spot your sample on it, then dip the paper into solvent. Make sure that pencil line stays above the solvent level, or your sample will wash away.
As the solvent travels up by capillary action, it carries the different substances at different speeds. The more soluble a substance is, the further it travels. This creates a beautiful pattern showing all the hidden components in your original mixture.
Pro tip: Always use pencil for the starting line because ink would interfere with your results by running up the paper too!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The chromatography setup follows three simple steps that even work brilliantly at home! First, you prepare your chromatography paper with a pencil line and carefully place dots of your sample (like black ink) on it.
Next comes the exciting bit - you lower the paper into a beaker with the right solvent, making sure the pencil line sits above the liquid. Then you just wait and watch as the solvent climbs up the paper, carrying different coloured substances at different rates.
Finally, you get to analyse your chromatogram! If you started with black ink, you might discover it's actually made from yellow, red, and blue components that have now separated into distinct bands.
Amazing fact: This technique can reveal the secret ingredients in everything from food colourings to crime scene evidence!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Filtration is your go-to method when you need to separate insoluble solids from liquids. Picture making coffee - the filter paper traps the coffee grounds whilst letting the liquid through. The trapped solid is called the residue, and the liquid that passes through is the filtrate.
Crystallisation works like magic to separate dissolved solids from solutions. You heat the solution to make water evaporate, leaving behind a saturated solution - basically, liquid that's holding as much dissolved solid as physically possible.
Here's a cool test: dip a clean, dry, cold glass rod into your heated solution. If crystals form on it when you pull it out, you've got a saturated solution! Let it cool slowly, and beautiful crystals will grow as the solid becomes less soluble in the cooler liquid.
Crystal care: Always wash your final crystals with distilled water and dry them properly between filter papers or in a drying oven.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Before diving into distillation, get these terms sorted: the solute is your dissolved solid, the solvent is the liquid doing the dissolving, and together they make a solution. Think tea again - sugar is the solute, water is the solvent, and sweet tea is your solution!
Simple distillation is brilliant for separating liquids from dissolved solids, like getting pure water from salty seawater. You heat the solution until the water evaporates and rises as vapour, then cool it back down in a condenser to collect pure liquid.
The process is beautifully simple - vapour travels through the condenser where cooling water flowing around it turns the vapour back into pure liquid. Meanwhile, all the dissolved salt gets left behind in the original container.
Temperature matters: Different substances have different boiling points, which is exactly what makes distillation work so well!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Fractional distillation is like simple distillation's clever cousin - it separates two or more liquids that mix together completely (called miscible liquids). Think separating alcohol from water, which is exactly how spirits are made!
The trick is using different boiling points. You heat the mixture to the temperature of whichever liquid boils first. For ethanol and water, ethanol boils at 78°C whilst water needs 100°C, so the ethanol evaporates first and gets collected separately.
The fractionating column makes this super efficient by providing extra surface area for separation. You stop heating when the temperature starts climbing towards the next liquid's boiling point - job done, perfectly separated liquids!
Real-world example: This is exactly how crude oil gets separated into petrol, diesel, and other useful products at refineries!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The story of atomic theory is like a detective story where each scientist built on previous discoveries! In 1803, John Dalton started it all with his atomic theory, suggesting matter was made of tiny, indestructible particles called atoms.
Dalton's key ideas were groundbreaking: atoms can't be created or destroyed, atoms of the same element are identical, and different atoms combine to make new substances. His "billiard ball" model pictured atoms as solid, indivisible spheres.
Fractional distillation continues with precise temperature control - when your ethanol and water mixture hits exactly 78°C, the ethanol boils off first. Stop heating when the temperature starts rising towards 100°C, and you've successfully separated them!
Science evolution: Dalton's theory was brilliant for its time, but science keeps improving as new evidence emerges!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
In 1897, J.J. Thomson completely revolutionised atomic theory by discovering the electron using a clever cathode-ray tube experiment. He showed that mysterious rays in the tube were actually streams of negatively charged particles - proving atoms weren't indivisible after all!
Thomson's experiment was ingenious: he created a beam of particles and watched them bend towards a positively charged plate. Since opposite charges attract, this proved the particles (electrons) were negatively charged. Dalton's "billiard ball" model was officially outdated!
This led to Thomson's "plum pudding" model - imagine a Christmas pudding where the pudding itself is positive and the raisins are negative electrons scattered throughout. It wasn't perfect, but it was a massive step forward in understanding atomic structure.
Game changer: Thomson's discovery proved that atoms have internal structure and aren't just solid balls!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Thomson's cathode-ray tube experiment was absolutely brilliant in its simplicity. He set up two metal plates - one positive, one negative - and watched as a mysterious beam curved towards the positive plate. This bending proved the beam was made of negatively charged particles.
The plum pudding model that emerged from this discovery pictured atoms as soft, positively charged material with electrons scattered throughout like raisins in a pudding. While we now know this isn't quite right, it was revolutionary for proving atoms had internal structure.
This discovery opened the floodgates for modern chemistry and physics. Thomson showed that atoms weren't the smallest particles after all - they contained even tinier subatomic particles that could be studied and understood.
Scientific method: Thomson's work perfectly shows how science progresses - each discovery builds on previous knowledge while sometimes overturning old ideas!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
5
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore essential laboratory techniques for separating mixtures, including simple distillation, fractional distillation, filtration, and chromatography. This summary covers the principles and processes involved in each method, highlighting key concepts such as boiling points and solubility. Ideal for students studying chemistry and separation methods.
Explore essential GCSE Chemistry practicals including chromatography, reaction rates, and water purification. Understand key concepts like mobile and stationary phases, R_f values, and the distillation process. This summary provides crucial information for mastering practical assessments and exam questions.
Explore key concepts in atomic structure, including isotopes, relative atomic mass, and the properties of elements and compounds. This summary also covers essential separation methods such as filtration, crystallization, and distillation, providing a comprehensive overview for AQA GCSE Chemistry paper 1 revision.
Explore the fundamentals of chemistry with this comprehensive overview of atoms, elements, compounds, and mixtures. This study note covers key concepts such as isotopes, relative atomic mass, separation techniques (evaporation, crystallization, chromatography, filtration, and distillation), and the historical development of atomic models. Ideal for AQA Triple Chemistry students preparing for exams.
Explore essential methods for separating mixtures, including filtration, crystallisation, and distillation. This summary covers the properties of mixtures, the nature of alloys, and detailed processes for effective separation. Ideal for chemistry students preparing for exams or seeking to understand key concepts in material science.
Exam board- AQA
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user