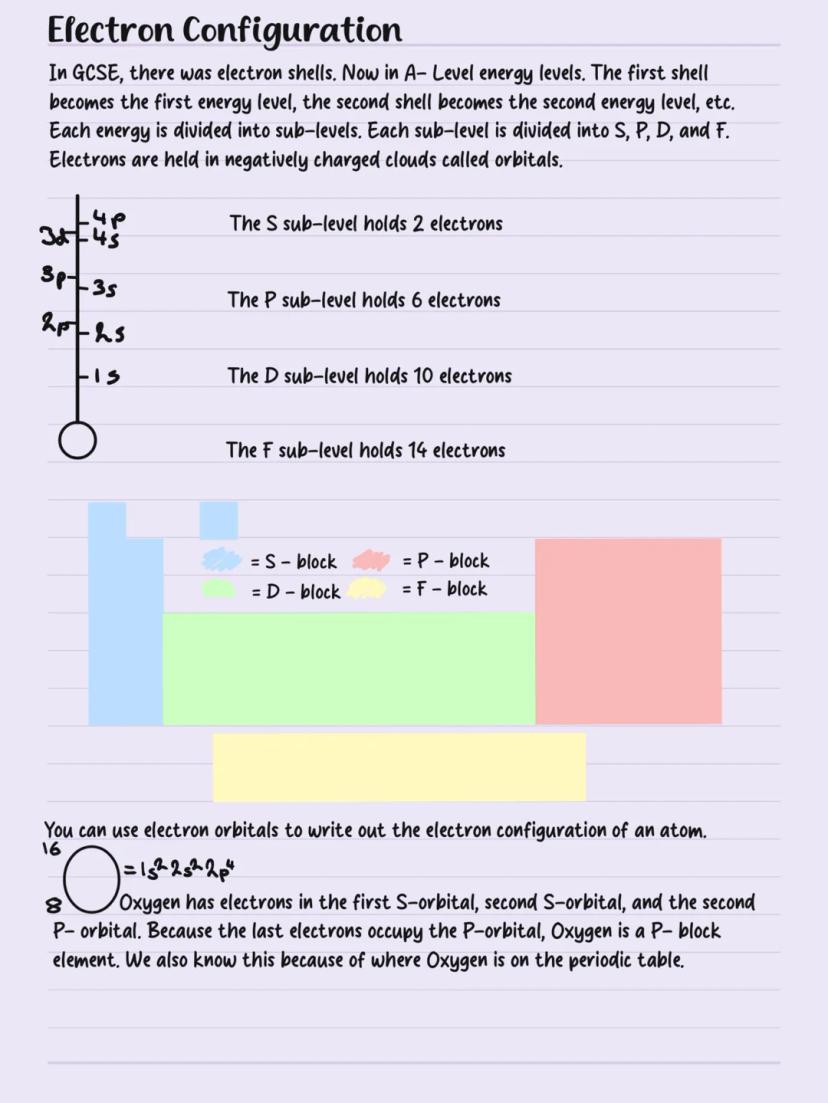
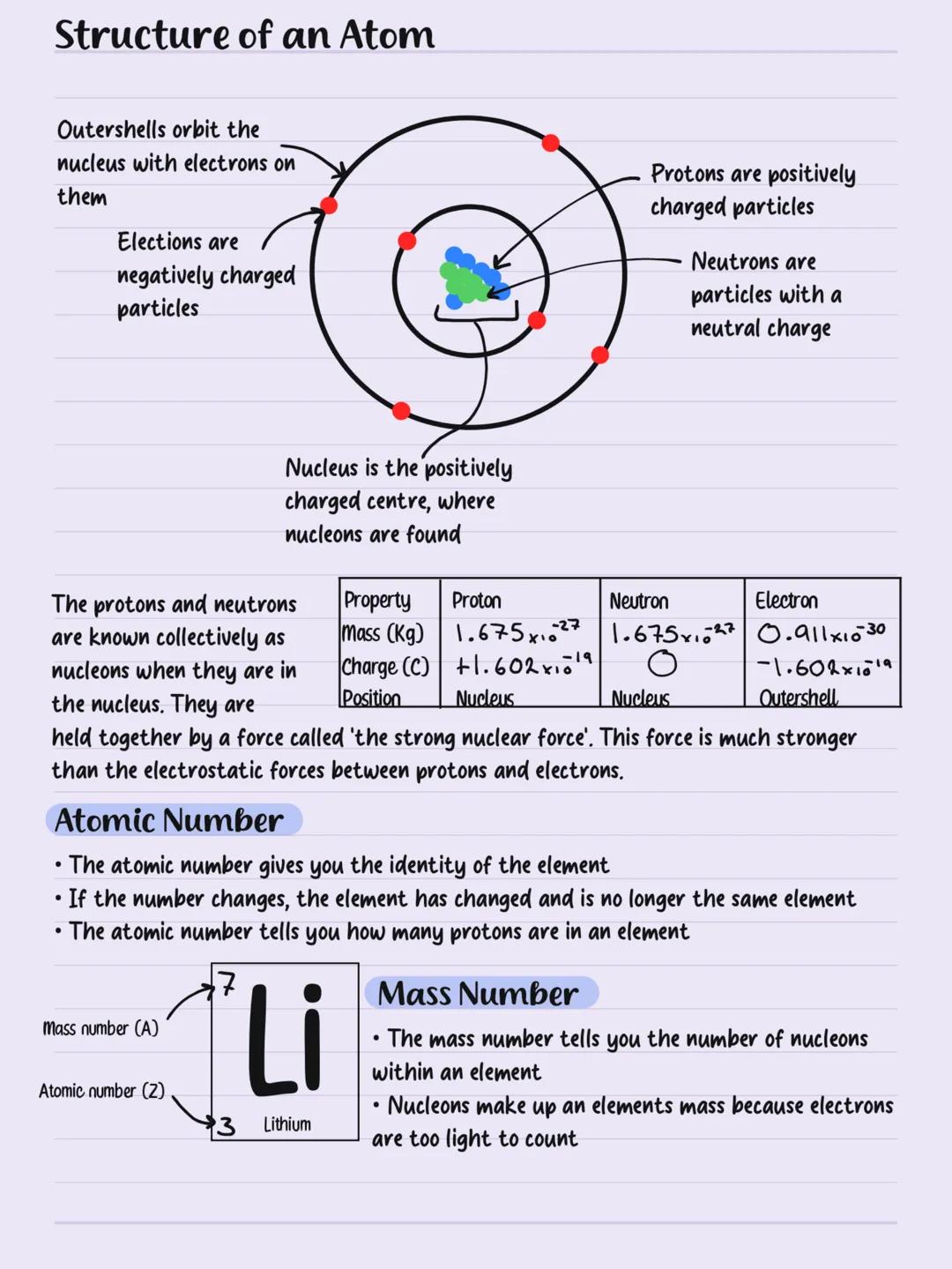
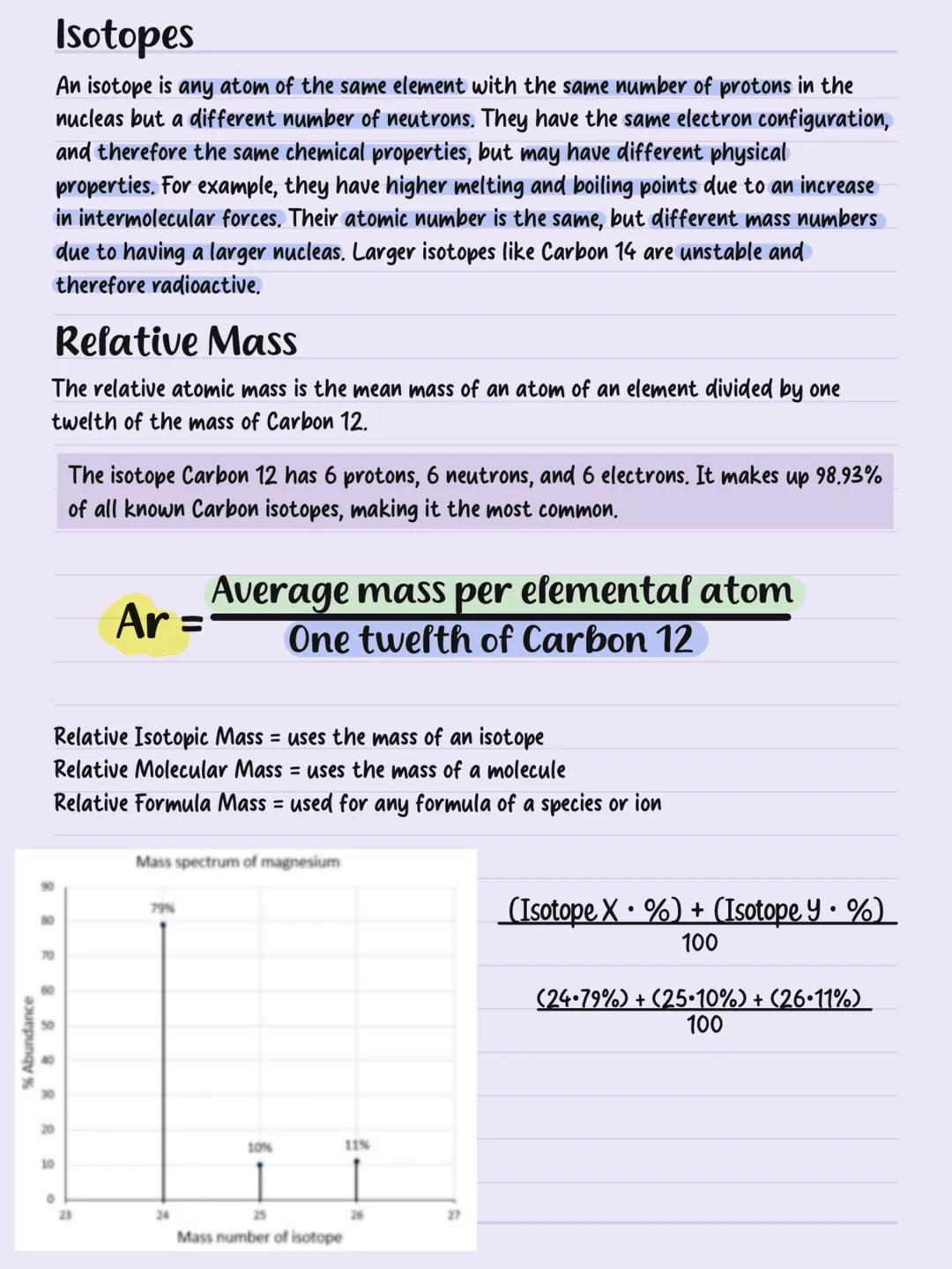
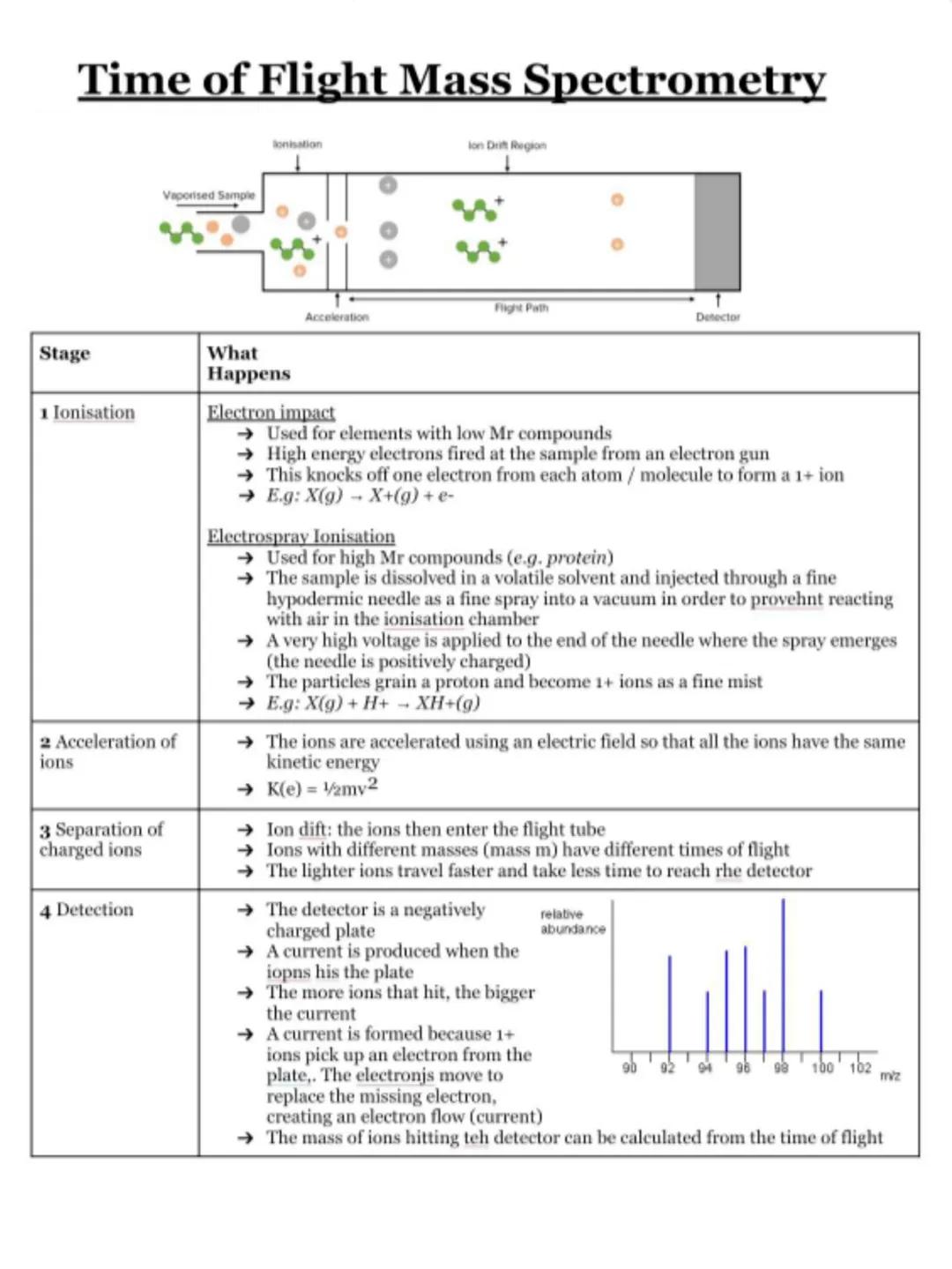
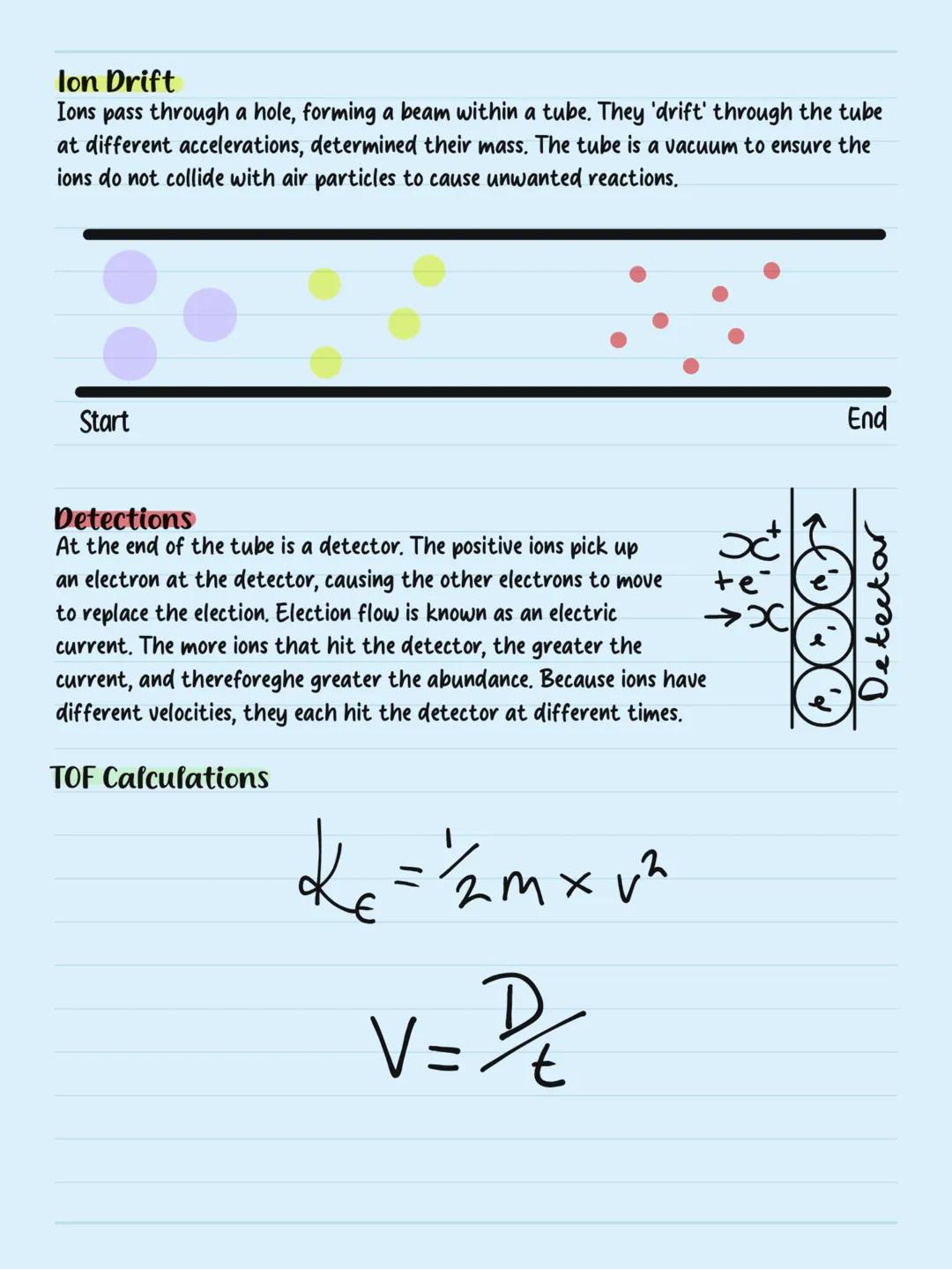
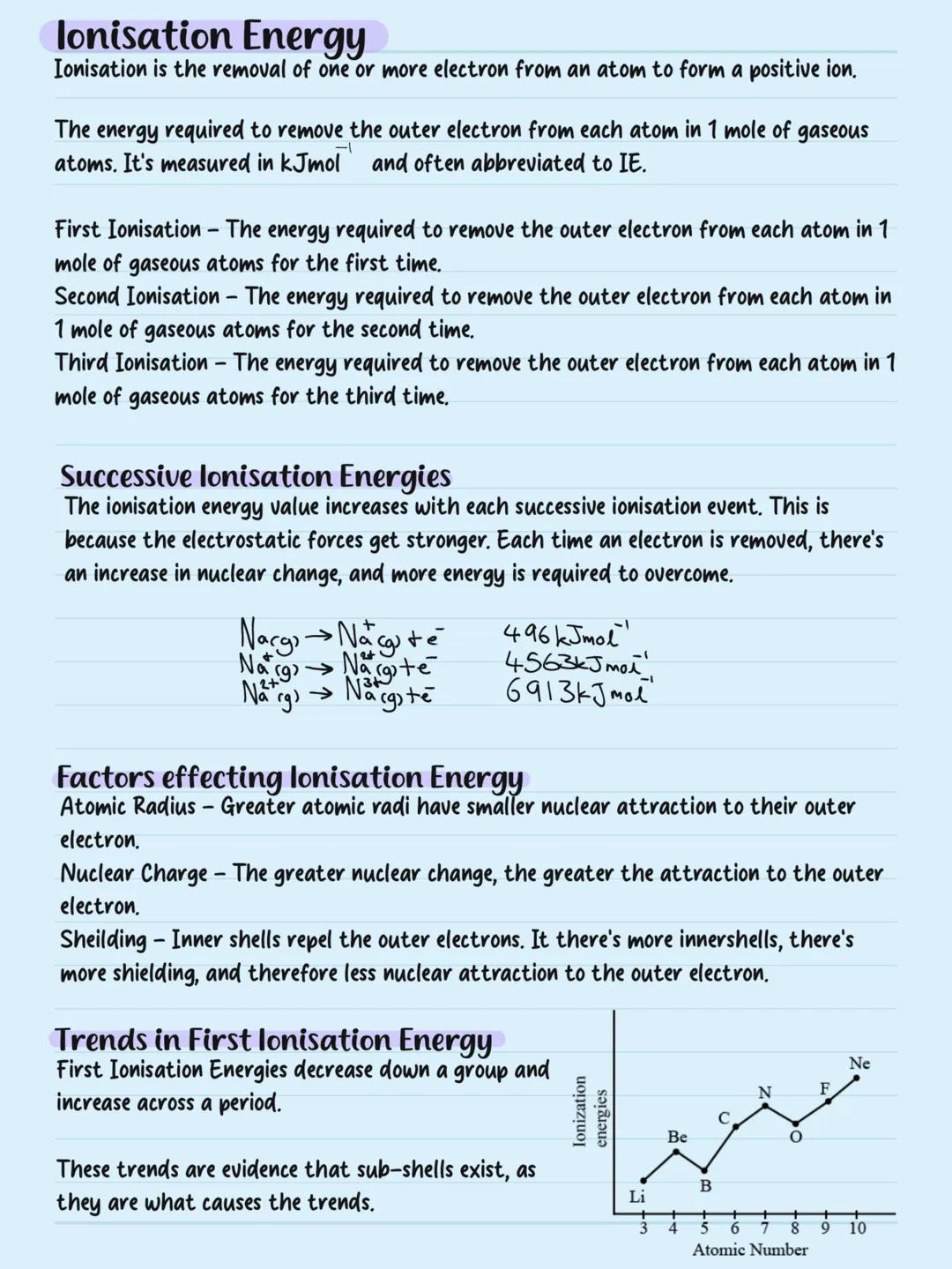
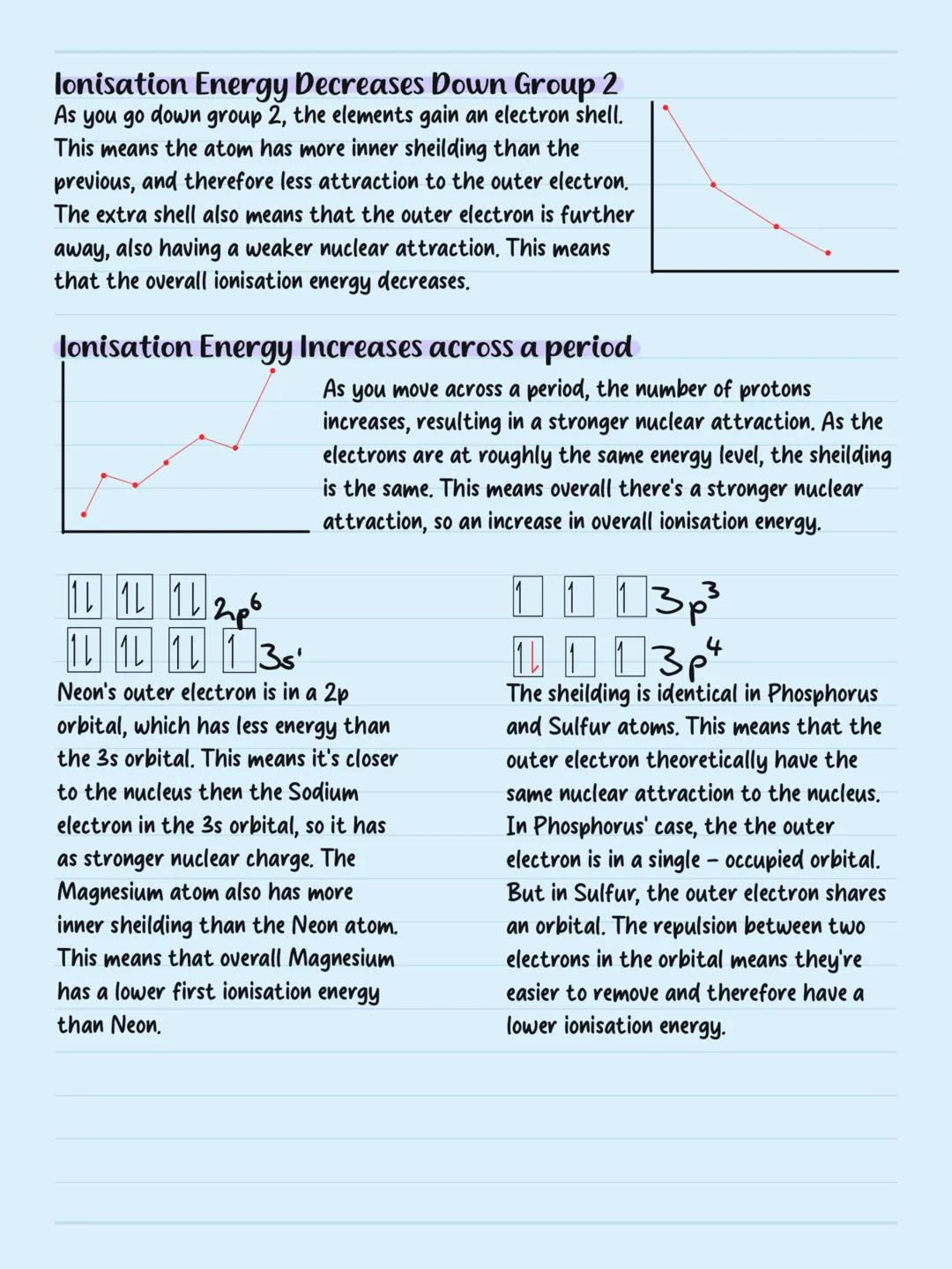
Electron Configuration
Forget GCSE electron shells - A-level uses energy levels divided into sub-levels (s, p, d, f). Think of it like a hotel with floors (energy levels) and different room types sub−levels holding specific numbers of guests (electrons).
Sub-level capacity is crucial: s holds 2 electrons, p holds 6, d holds 10, and f holds 14. Electrons live in orbitals - regions of space around the nucleus.
Writing electron configuration shows where electrons live. Oxygen (8 electrons) is 1s²2s²2p⁴. Since oxygen's outermost electrons occupy p-orbitals, it's a p-block element - matching its position on the periodic table.
Memory Trick: Remember "2, 6, 10, 14" for s, p, d, f capacities - it's the foundation for all electron configurations.