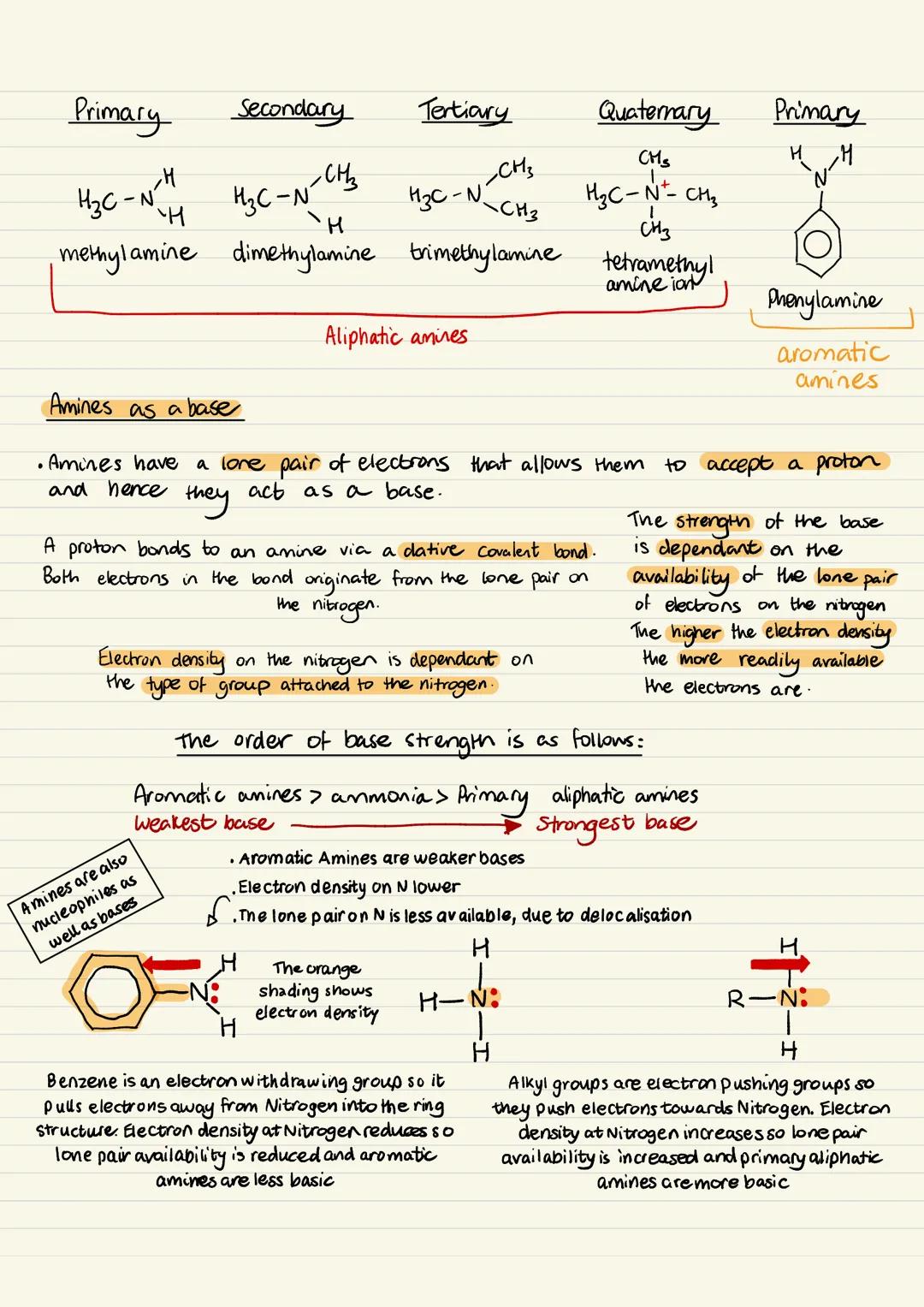
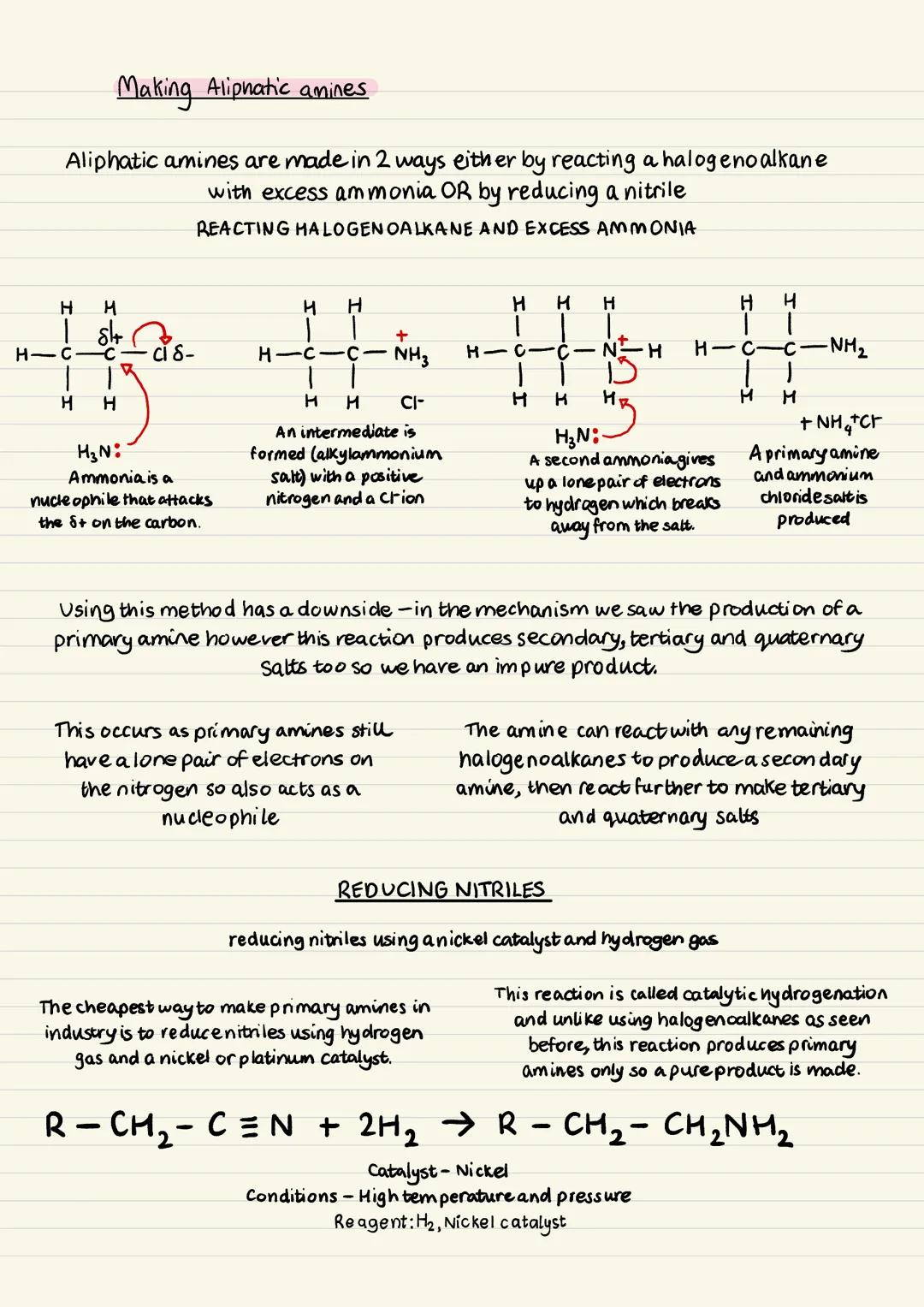
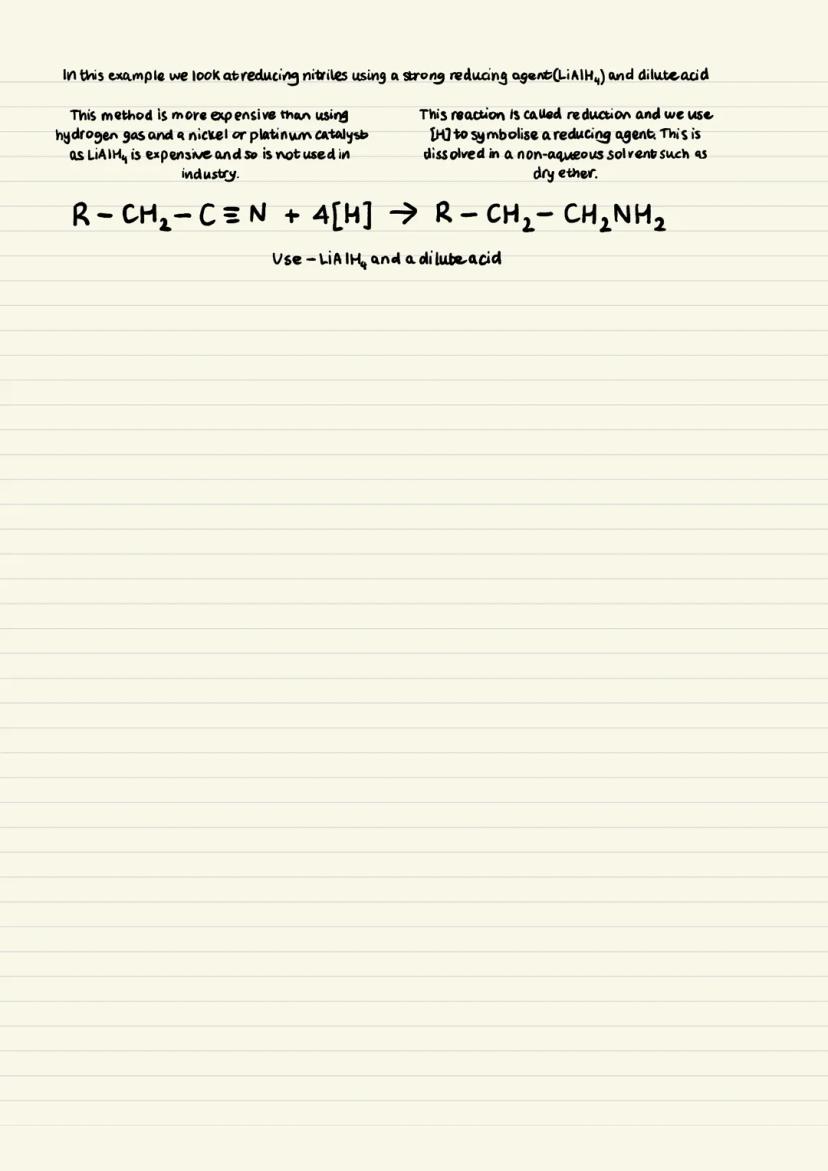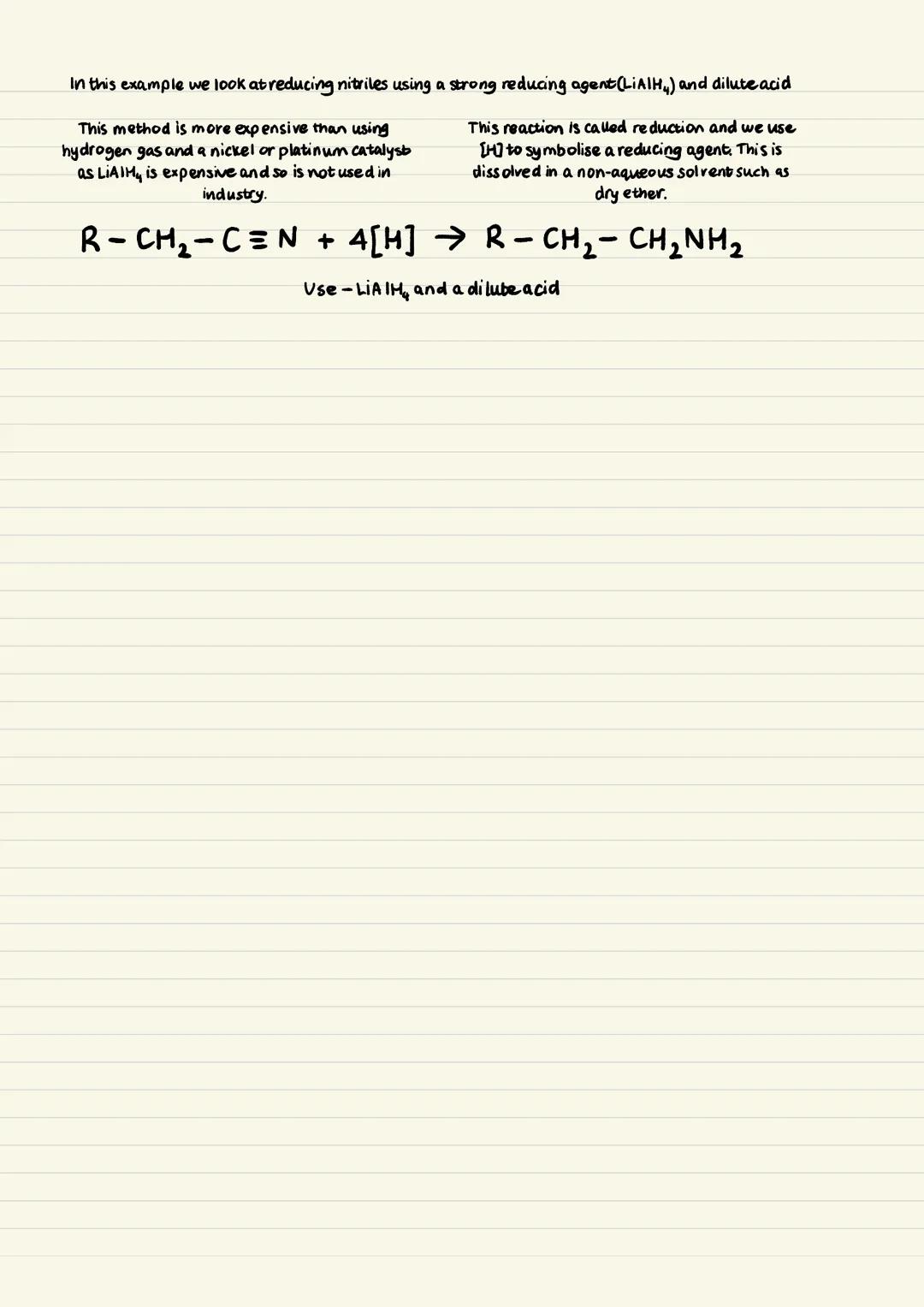Making Aliphatic Amines - Method 1
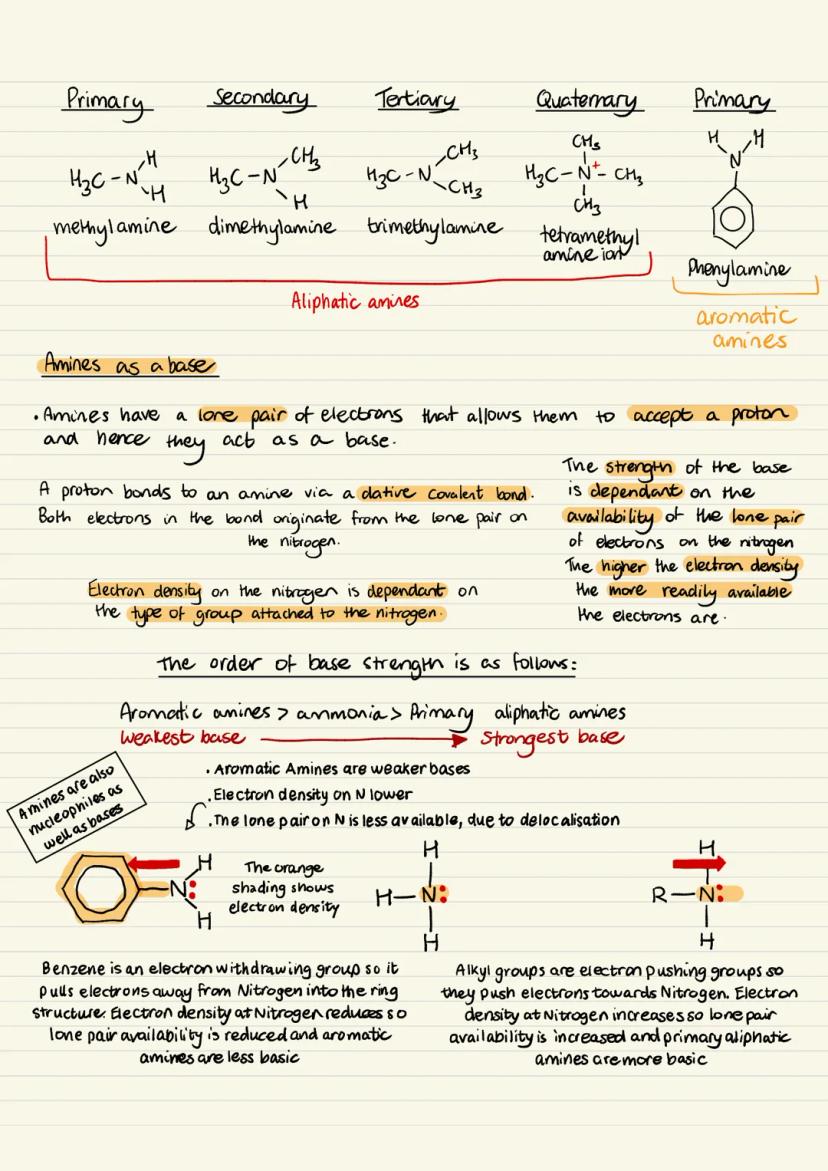
There are two main ways to synthesise aliphatic amines in the lab and industry. The first involves reacting halogenoalkanes with excess ammonia.
In this mechanism, ammonia acts as a nucleophile and attacks the carbon attached to the halogen. This forms an alkylammonium salt intermediate with a positively charged nitrogen and a chloride ion.
A second ammonia molecule then removes a hydrogen from this salt, producing the primary amine and ammonium chloride. However, this method has a major drawback - it produces a mixture of primary, secondary, tertiary amines, and quaternary salts.
This happens because the primary amine still has lone pairs and can act as a nucleophile itself, reacting with more halogenoalkane molecules. That's why you get an impure product that needs separation.
Exam Tip: Always mention the impurity problem when discussing the halogenoalkane method - it's a common exam question!