Diving into science can be both exciting and challenging. This... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
1,041
•
28 Jan 2026
•
jaeden
@jaedenc08
Diving into science can be both exciting and challenging. This... Show more
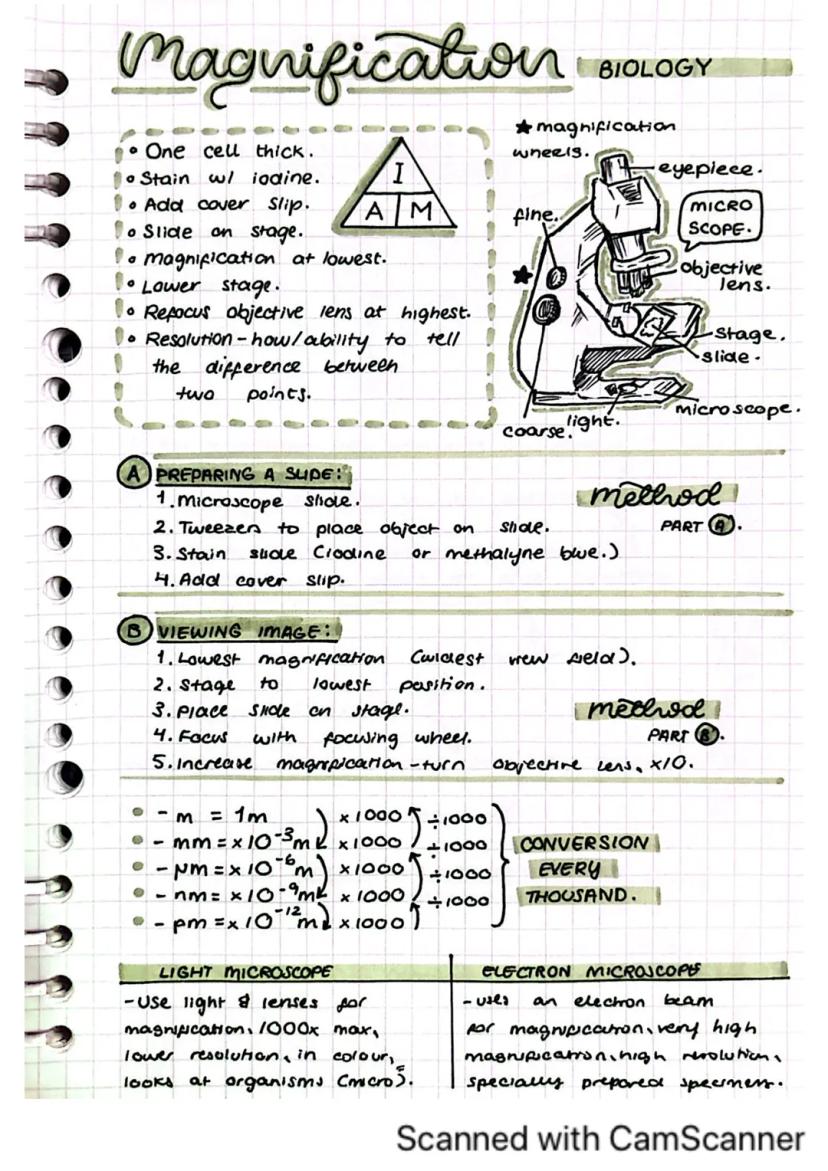



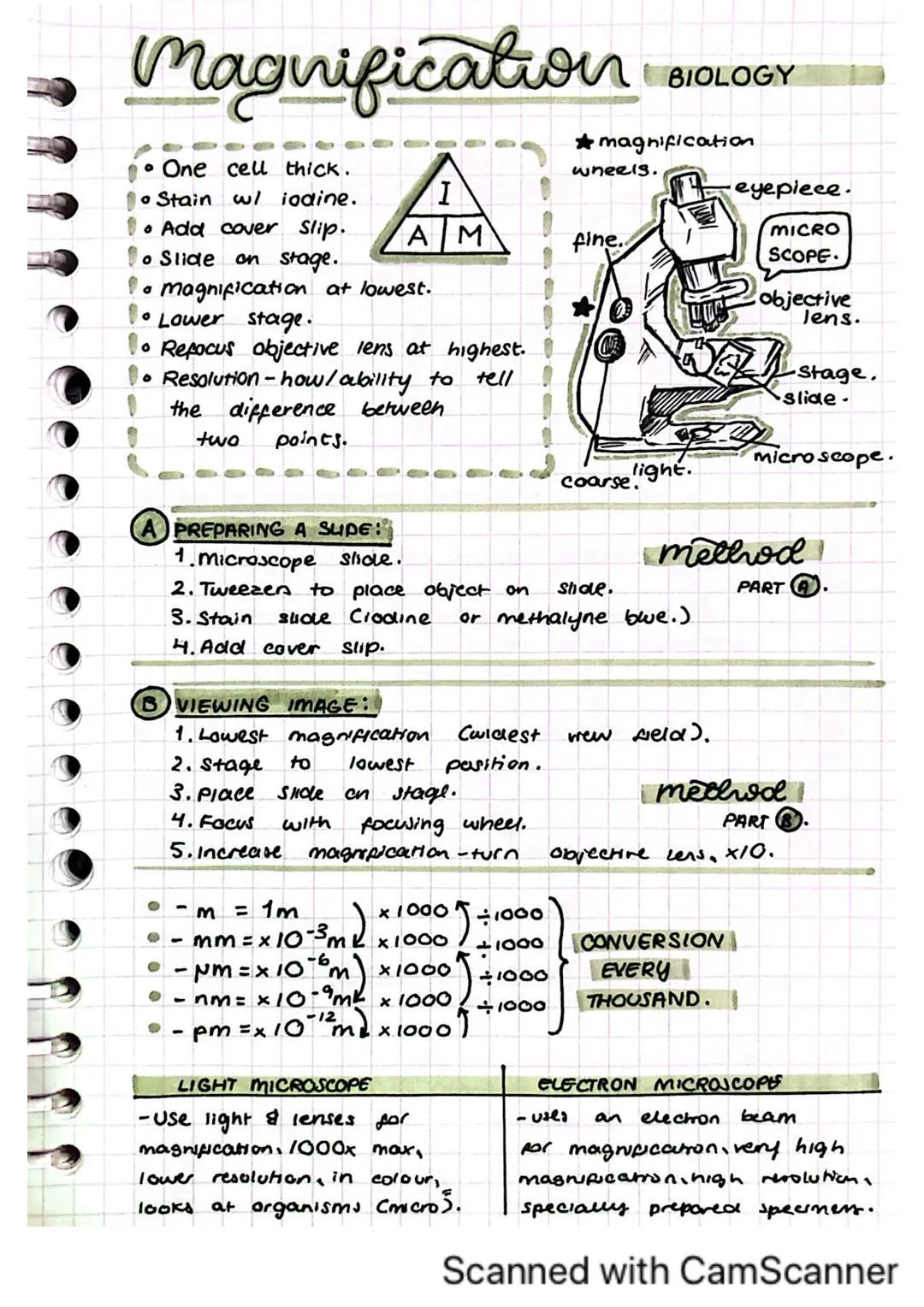
Microscopes let you see the tiny world that's invisible to our eyes. Knowing how to use one properly is a crucial lab skill you'll use throughout your science education.
To prepare a slide properly, place your specimen on a microscope slide, add a stain like iodine or methylene blue, and carefully place a cover slip on top. This creates a thin, viewable sample that's just one cell thick.
When viewing your sample, always start with the lowest magnification. Place your slide on the stage, lower it to its lowest position, and focus using the coarse adjustment wheel first, then the fine adjustment for clearer detail.
Quick Tip: Remember the scale conversions: 1mm = 10⁻³m, 1μm = 10⁻⁶m, 1nm = 10⁻⁹m. Each step is 1,000 times smaller than the previous!
Light microscopes use light and lenses for magnification (up to 1000×) and show specimens in colour. Electron microscopes use electron beams for much higher magnification and resolution, though images are not in colour and require specially prepared specimens.

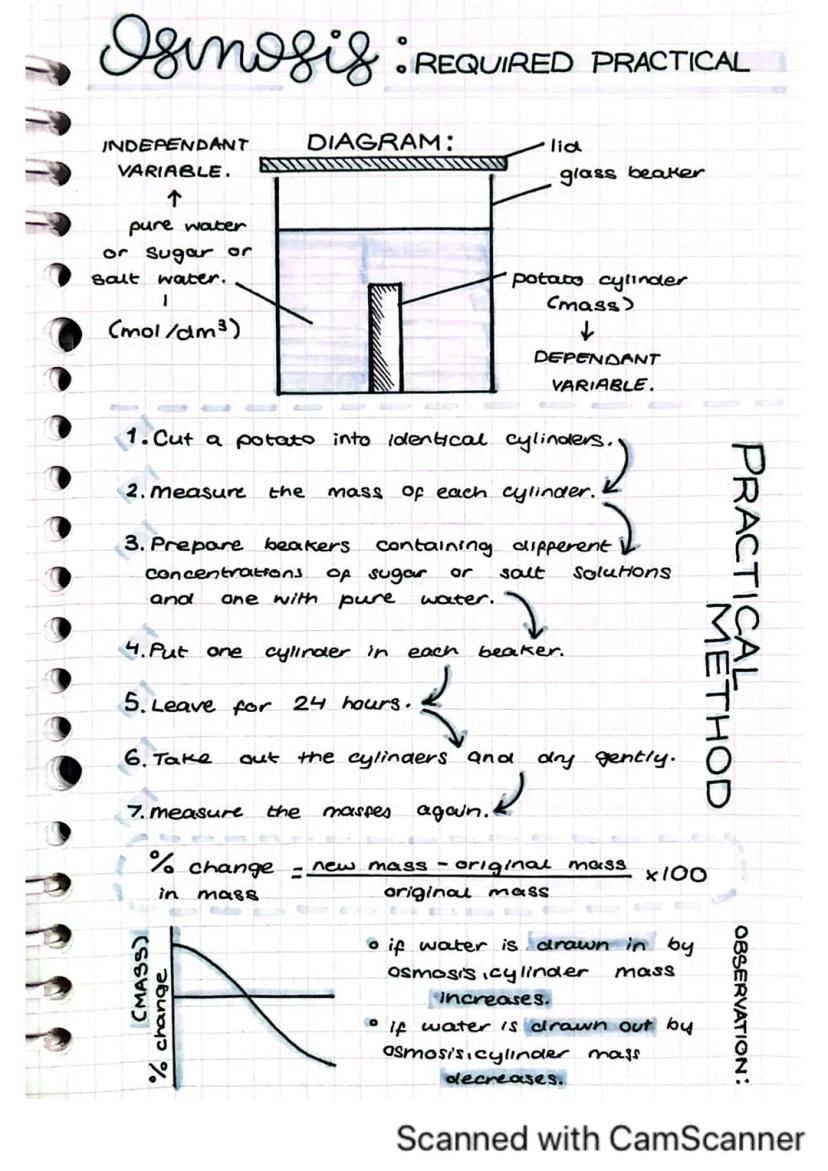
Osmosis is the movement of water molecules across a partially permeable membrane. This practical helps you see this process in action using potato cylinders.
The method is straightforward: cut identical potato cylinders, weigh them, and place them in beakers containing different concentrations of sugar or salt solutions (plus one with pure water). After 24 hours, remove the cylinders, dry them gently, and weigh them again to calculate the percentage change in mass.
When water moves into a potato cylinder by osmosis, its mass increases. Conversely, when water moves out of the cylinder, the mass decreases. This change tells you about the direction of water movement.
Remember: The percentage change in mass is calculated using the formula: % change = ÷ original mass × 100
In this experiment, the concentration of solution is your independent variable, while the percentage change in mass is your dependent variable. This practical demonstrates how osmosis works in real biological tissues.
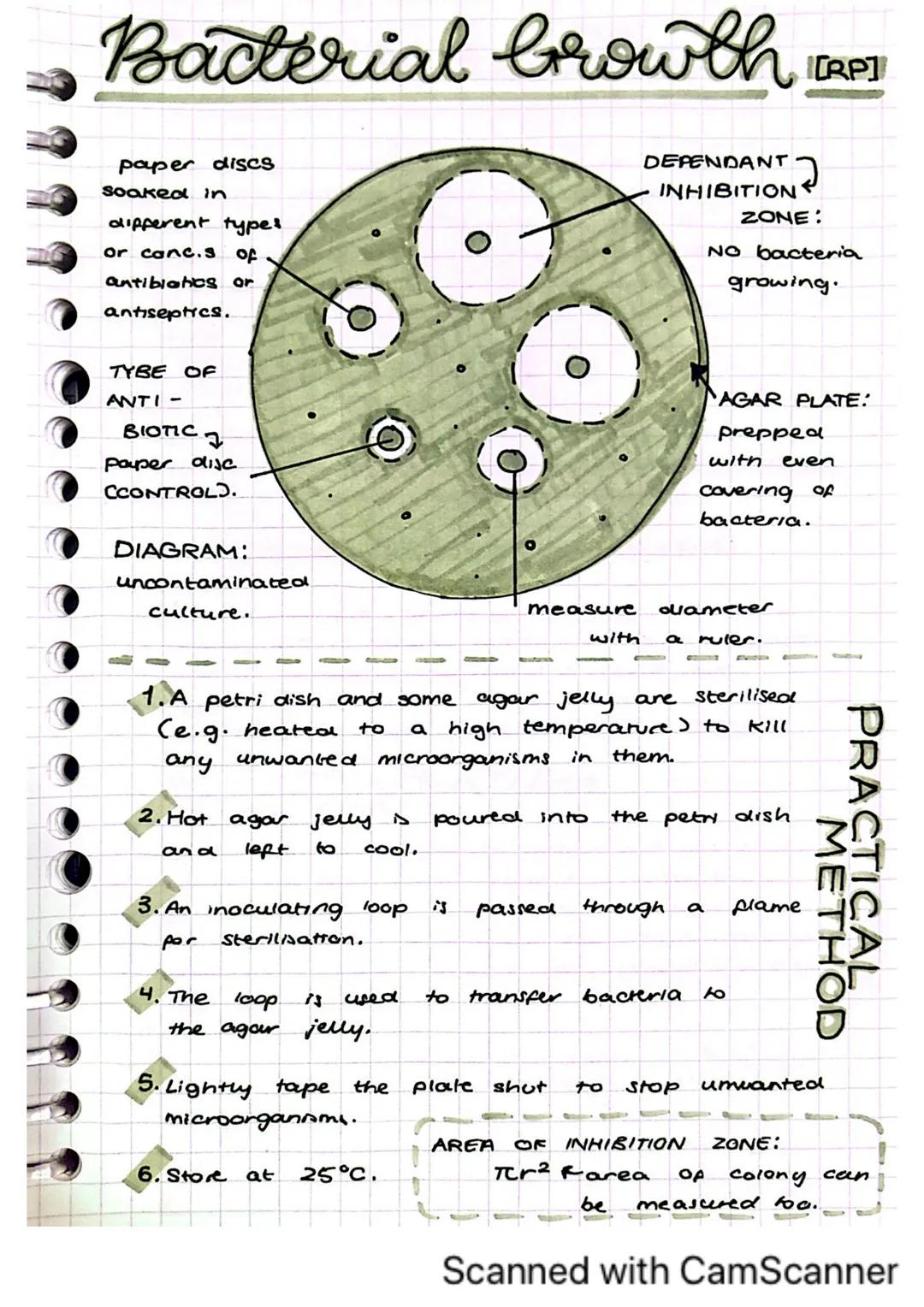
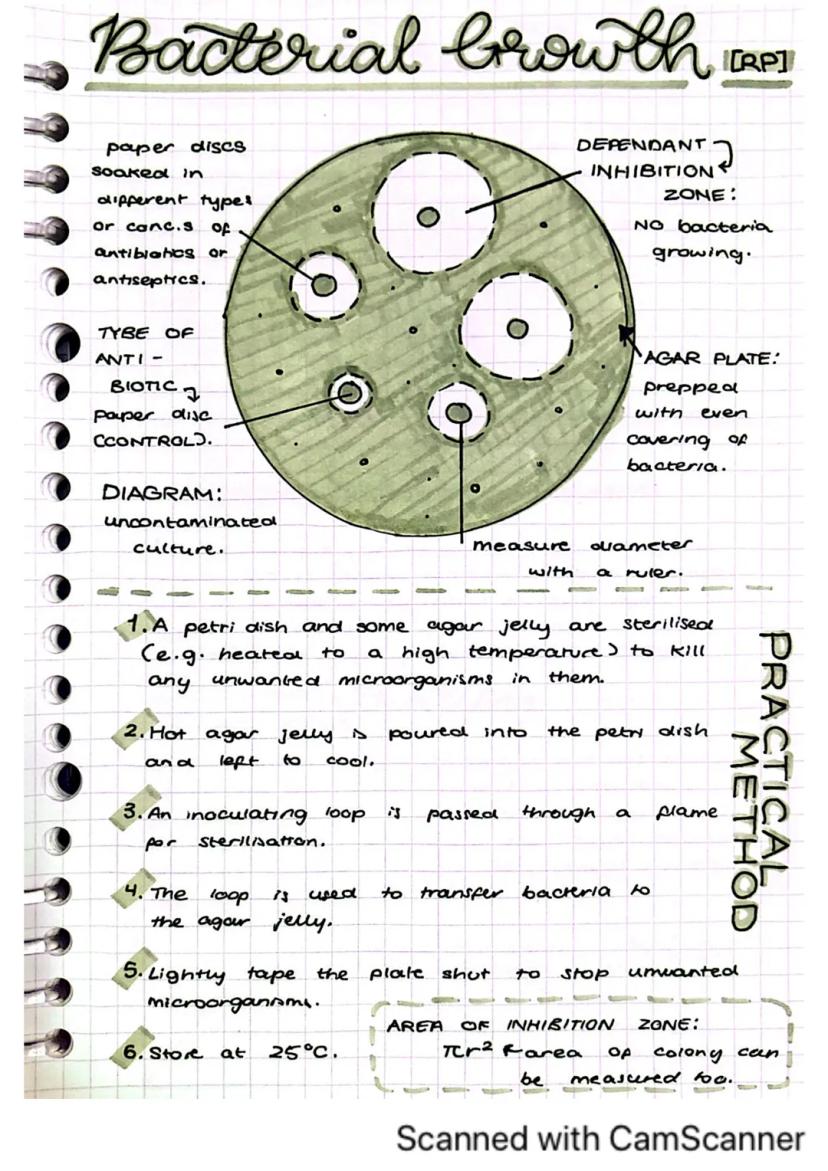
This practical helps you understand how antibiotics and antiseptics affect bacterial growth, which is essential knowledge for medicine and health.
The setup involves an agar plate prepared with an even coverage of bacteria. Paper discs soaked in different types or concentrations of antibiotics or antiseptics are placed on the plate. The plate is then sealed and incubated at 25°C.
After incubation, you'll observe zones of inhibition around the paper discs where bacteria couldn't grow. The larger this clear zone, the more effective the antibiotic is against the bacteria. You can measure the diameter of these zones with a ruler.
Did you know? You can calculate the area of the inhibition zone using πr² (where r is the radius), which gives a more accurate measurement of antibiotic effectiveness than just measuring the diameter.
This practical requires careful sterile technique to avoid contamination. The type of antibiotic is your independent variable, while the size of the inhibition zone is your dependent variable.

Learning to culture microorganisms safely is a fundamental skill in microbiology. It allows you to study bacteria and test treatments against them.
Aseptic techniques are essential to prevent unwanted microorganisms from contaminating your culture. This includes sterilizing equipment by heating (like passing an inoculating loop through a flame) before use. Your culture medium typically contains agar with nutrients and glucose to feed the bacteria.
When testing antibiotics, you'll observe zones of inhibition where bacteria can't grow. A larger zone indicates a more effective antibiotic. If there's no zone, the bacteria may have developed resistance to that antibiotic.
Lab Safety: Always incubate your cultures at 25°C (or 37°C in labs for more rapid growth) and keep the petri dishes sealed and upside down to prevent contamination.
Common sources of error include improper spacing of antibiotic disks and contamination from poor aseptic technique. Remember your variables: independent (type of antibiotic), dependent (size of the inhibition zone), and controlled .

Ecological field work lets you study organisms in their natural environment. It's a hands-on way to understand how living things interact with their surroundings.
When investigating something like the effect of trampling on plant growth, you might form a hypothesis: "Areas with more trampling will have fewer daisy plants than untrampled areas." To test this, you'd use a quadrat (a square frame) placed randomly on the ground to count plants within that area.
For systematic sampling, you can use a transect - a line across an area where measurements are taken at regular intervals. This is great for studying changes across boundaries, like from a footpath to untrampled grass.
Safety First: Always wash your hands after ecology work, and when throwing quadrats, throw them low to avoid accidents.
Remember to identify your variables clearly: independent variable , dependent variable (number of plants, percentage coverage, abundance score). Repeat your sampling multiple times to calculate reliable means and eliminate bias from your results.

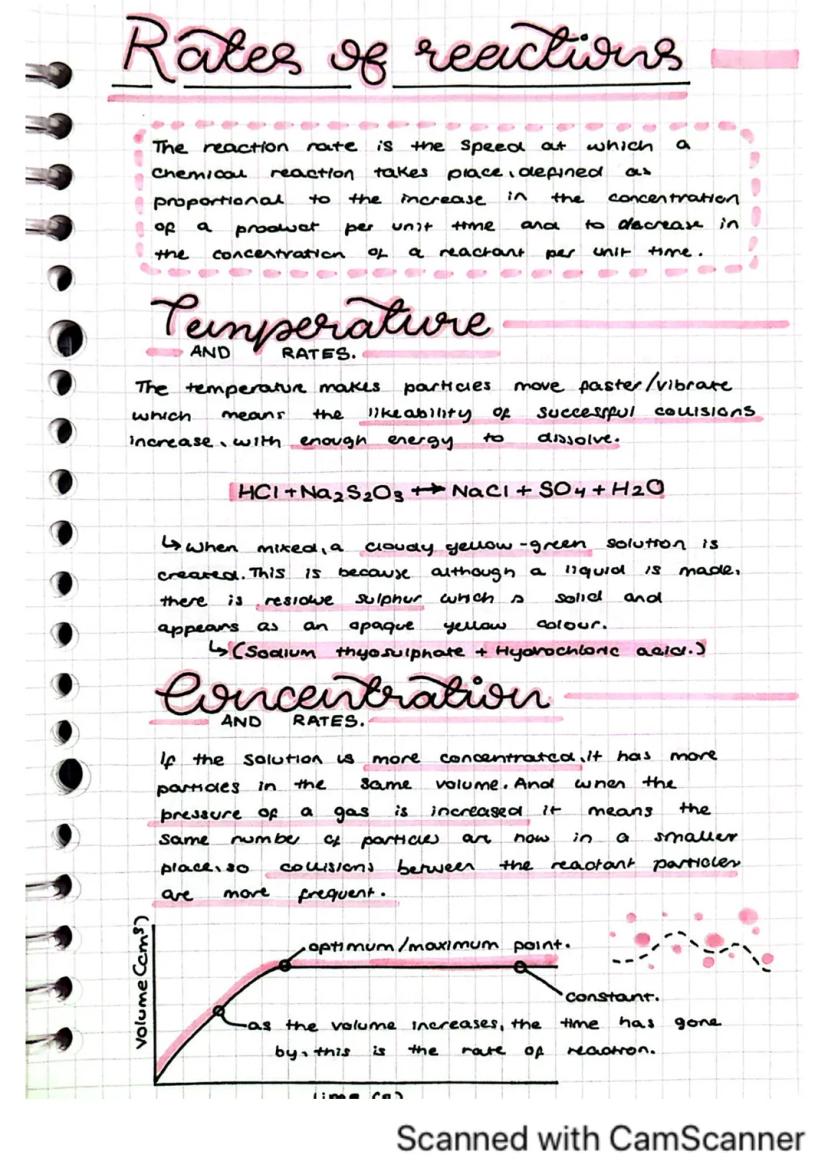
Chemical reactions happen at different speeds depending on certain conditions. Understanding reaction rates is crucial for controlling chemical processes in industry and research.
Temperature significantly affects reaction rates. When you increase temperature, particles move faster and vibrate more energetically, leading to more frequent and more energetic collisions. This increases the likelihood of successful collisions with enough energy to cause a reaction.
Concentration also plays a vital role in reaction rates. A more concentrated solution has more particles in the same volume, resulting in more frequent collisions between reactant particles. Similarly, increasing gas pressure means particles are closer together, also increasing collision frequency.
Try This: In the reaction between sodium thiosulphate and hydrochloric acid , you can observe the rate by timing how long it takes for the solution to turn cloudy yellow-green due to sulphur formation.
As a reaction progresses, you can measure how the volume or mass changes over time. The rate of these changes indicates the reaction rate - faster changes mean faster reactions.

Chemical equilibrium is a fascinating state where forward and reverse reactions occur at exactly the same rate. It's like a chemical tug-of-war that results in a perfect balance.
There are different types of systems in chemistry: open systems release both mass and heat, closed systems release only heat, and isolated systems release nothing at all. Most equilibrium reactions we study occur in closed systems.
Le Chatelier's principle is crucial to understand: if a change is made to a system at equilibrium, the equilibrium position will shift to oppose that change. For example, if you add more reactants, the equilibrium shifts to use them up; if you remove products, it shifts to make more.
Visual Example: Hydrated copper sulphate (blue) and anhydrous copper sulphate (white) exist in equilibrium. Adding water shifts to the blue form; heating shifts to the white form.
In a dynamic equilibrium, the forward and reverse reactions continue to occur at equal rates, so there's no overall change in the amounts of reactants and products. This happens in reactions like CO₂(g) ⇌ CO₂(aq) and 2H₂ + O₂ ⇌ 2H₂O.
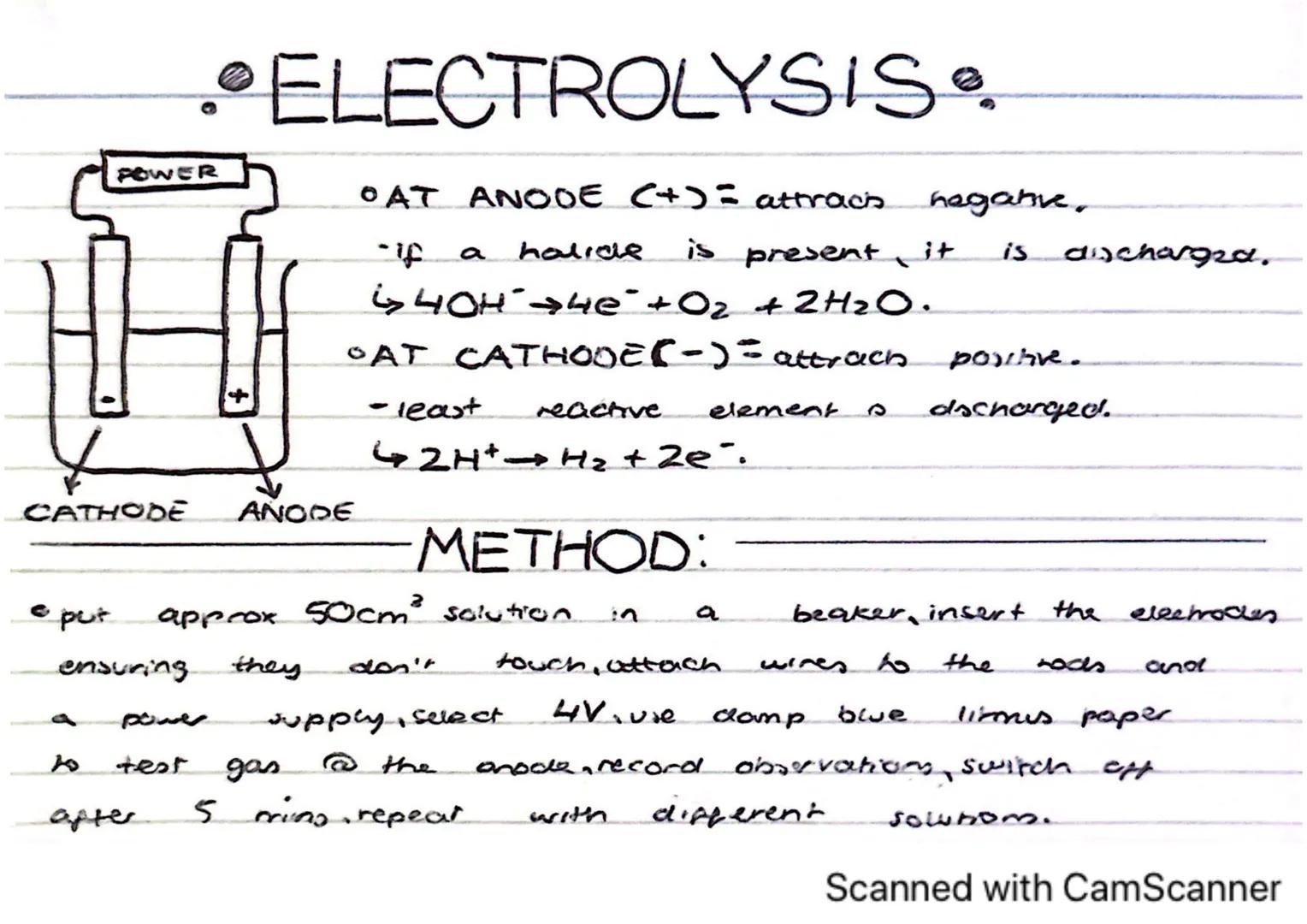
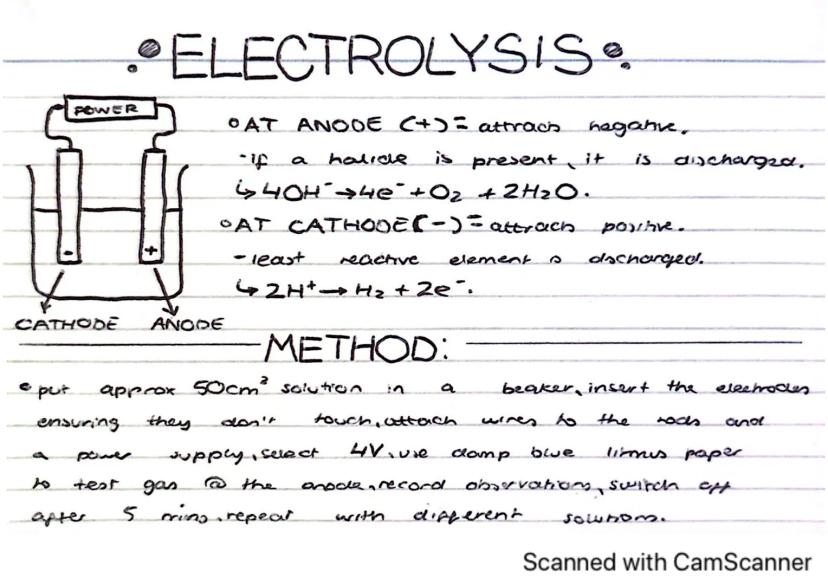
Electrolysis is a fascinating process that uses electrical current to drive chemical reactions that wouldn't happen spontaneously. It's how we extract reactive metals and produce many chemicals industrially.
At the anode (positive electrode), negatively charged ions are attracted and discharged. If halide ions (like chloride) are present, they're discharged. Otherwise, hydroxide ions (OH⁻) are discharged following the reaction: 4OH⁻ → 4e⁻ + O₂ + 2H₂O.
At the cathode (negative electrode), positively charged ions are attracted. The least reactive element will be discharged first. In solutions with hydrogen ions, you'll see: 2H⁺ + 2e⁻ → H₂.
Practical Tip: You can test for oxygen gas produced at the anode using damp blue litmus paper - it will bleach in the presence of oxygen.
To perform electrolysis, pour about 50cm³ of solution into a beaker, insert electrodes making sure they don't touch, connect to a 4V power supply, and run for about 5 minutes while making observations. Repeat with different solutions to compare results.
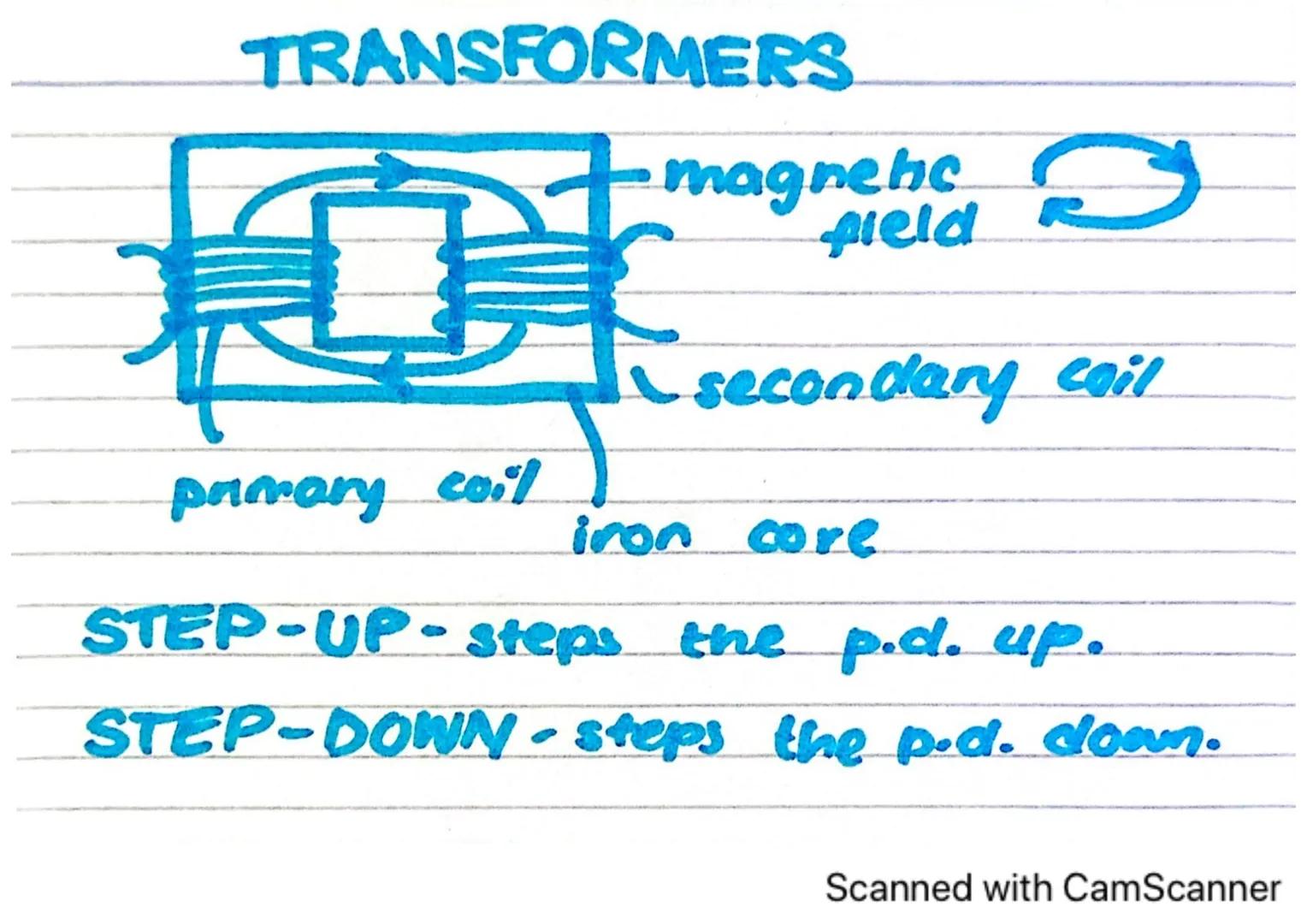
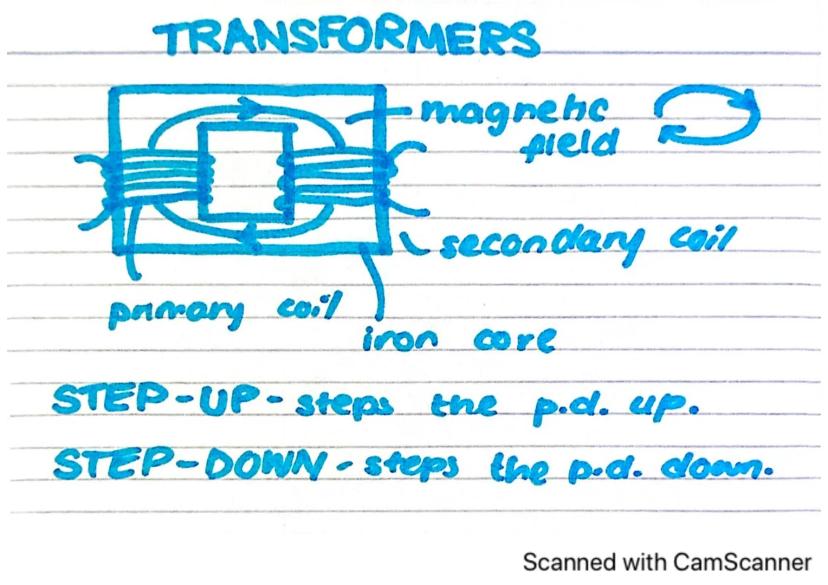
Transformers are clever devices that can change the voltage of alternating current electricity without wasting much energy. They're essential for our national electricity grid.
A transformer consists of two coils of wire (primary and secondary) wrapped around an iron core. When alternating current flows through the primary coil, it creates a changing magnetic field in the iron core. This changing field then induces a voltage in the secondary coil.
There are two main types of transformers: step-up transformers increase the voltage, while step-down transformers decrease it. The relationship between the voltages depends on the number of turns in each coil.
Remember: Transformers only work with alternating current (AC), never with direct current (DC), because you need a changing magnetic field to induce voltage in the secondary coil.
Transformers are based on electromagnetic induction, discovered by Michael Faraday. This principle is used throughout our electrical distribution system to efficiently deliver power to homes and businesses.
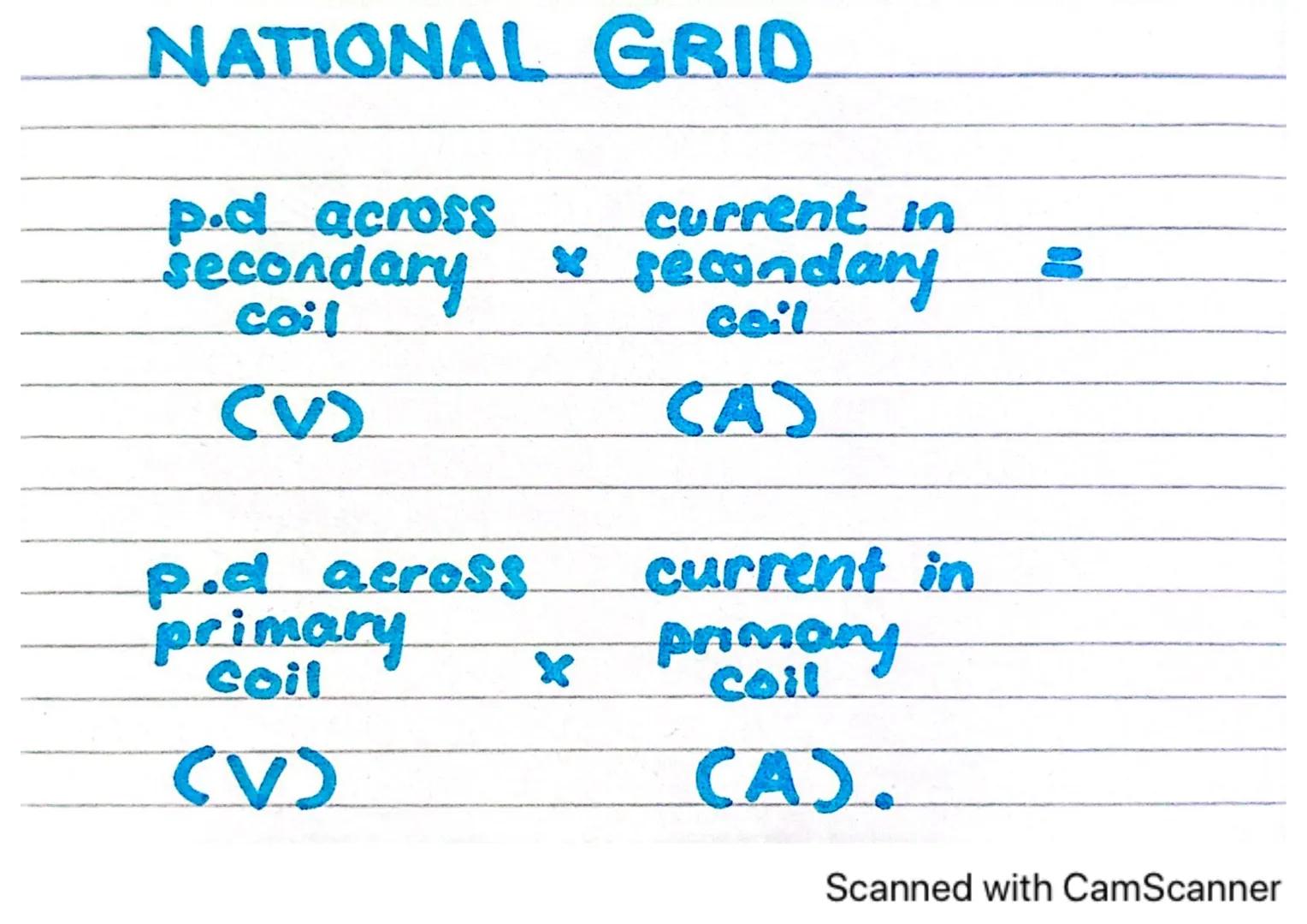
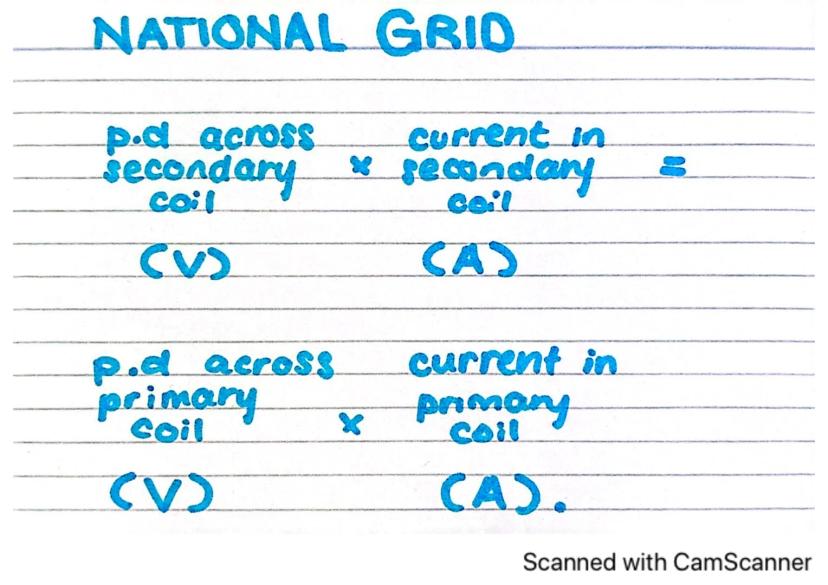
The National Grid is the network that distributes electricity across the country. It relies heavily on transformers to work efficiently.
Transformers in the National Grid follow a simple mathematical relationship: the ratio of voltages equals the inverse ratio of currents. If we increase the voltage across the secondary coil compared to the primary coil, we decrease the current in the secondary coil proportionally.
This relationship is crucial for efficient power transmission. By stepping up the voltage to very high levels for transmission over long distances, we reduce the current, which dramatically reduces energy losses due to heating in the wires.
Power Fact: The voltage in the National Grid's transmission lines can be as high as 400,000 volts, but this is stepped down in stages before reaching the 230V used in UK homes.
Near power stations, step-up transformers increase voltage for transmission. Then, step-down transformers at substations gradually reduce the voltage as electricity gets closer to homes and businesses, making it safe for domestic use.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
jaeden
@jaedenc08
Diving into science can be both exciting and challenging. This summary covers key practical methods, concepts, and techniques from biology, chemistry, and physics that you'll need to understand for your coursework and exams. Each section provides essential information in bite-sized... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Microscopes let you see the tiny world that's invisible to our eyes. Knowing how to use one properly is a crucial lab skill you'll use throughout your science education.
To prepare a slide properly, place your specimen on a microscope slide, add a stain like iodine or methylene blue, and carefully place a cover slip on top. This creates a thin, viewable sample that's just one cell thick.
When viewing your sample, always start with the lowest magnification. Place your slide on the stage, lower it to its lowest position, and focus using the coarse adjustment wheel first, then the fine adjustment for clearer detail.
Quick Tip: Remember the scale conversions: 1mm = 10⁻³m, 1μm = 10⁻⁶m, 1nm = 10⁻⁹m. Each step is 1,000 times smaller than the previous!
Light microscopes use light and lenses for magnification (up to 1000×) and show specimens in colour. Electron microscopes use electron beams for much higher magnification and resolution, though images are not in colour and require specially prepared specimens.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Osmosis is the movement of water molecules across a partially permeable membrane. This practical helps you see this process in action using potato cylinders.
The method is straightforward: cut identical potato cylinders, weigh them, and place them in beakers containing different concentrations of sugar or salt solutions (plus one with pure water). After 24 hours, remove the cylinders, dry them gently, and weigh them again to calculate the percentage change in mass.
When water moves into a potato cylinder by osmosis, its mass increases. Conversely, when water moves out of the cylinder, the mass decreases. This change tells you about the direction of water movement.
Remember: The percentage change in mass is calculated using the formula: % change = ÷ original mass × 100
In this experiment, the concentration of solution is your independent variable, while the percentage change in mass is your dependent variable. This practical demonstrates how osmosis works in real biological tissues.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This practical helps you understand how antibiotics and antiseptics affect bacterial growth, which is essential knowledge for medicine and health.
The setup involves an agar plate prepared with an even coverage of bacteria. Paper discs soaked in different types or concentrations of antibiotics or antiseptics are placed on the plate. The plate is then sealed and incubated at 25°C.
After incubation, you'll observe zones of inhibition around the paper discs where bacteria couldn't grow. The larger this clear zone, the more effective the antibiotic is against the bacteria. You can measure the diameter of these zones with a ruler.
Did you know? You can calculate the area of the inhibition zone using πr² (where r is the radius), which gives a more accurate measurement of antibiotic effectiveness than just measuring the diameter.
This practical requires careful sterile technique to avoid contamination. The type of antibiotic is your independent variable, while the size of the inhibition zone is your dependent variable.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Learning to culture microorganisms safely is a fundamental skill in microbiology. It allows you to study bacteria and test treatments against them.
Aseptic techniques are essential to prevent unwanted microorganisms from contaminating your culture. This includes sterilizing equipment by heating (like passing an inoculating loop through a flame) before use. Your culture medium typically contains agar with nutrients and glucose to feed the bacteria.
When testing antibiotics, you'll observe zones of inhibition where bacteria can't grow. A larger zone indicates a more effective antibiotic. If there's no zone, the bacteria may have developed resistance to that antibiotic.
Lab Safety: Always incubate your cultures at 25°C (or 37°C in labs for more rapid growth) and keep the petri dishes sealed and upside down to prevent contamination.
Common sources of error include improper spacing of antibiotic disks and contamination from poor aseptic technique. Remember your variables: independent (type of antibiotic), dependent (size of the inhibition zone), and controlled .

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ecological field work lets you study organisms in their natural environment. It's a hands-on way to understand how living things interact with their surroundings.
When investigating something like the effect of trampling on plant growth, you might form a hypothesis: "Areas with more trampling will have fewer daisy plants than untrampled areas." To test this, you'd use a quadrat (a square frame) placed randomly on the ground to count plants within that area.
For systematic sampling, you can use a transect - a line across an area where measurements are taken at regular intervals. This is great for studying changes across boundaries, like from a footpath to untrampled grass.
Safety First: Always wash your hands after ecology work, and when throwing quadrats, throw them low to avoid accidents.
Remember to identify your variables clearly: independent variable , dependent variable (number of plants, percentage coverage, abundance score). Repeat your sampling multiple times to calculate reliable means and eliminate bias from your results.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemical reactions happen at different speeds depending on certain conditions. Understanding reaction rates is crucial for controlling chemical processes in industry and research.
Temperature significantly affects reaction rates. When you increase temperature, particles move faster and vibrate more energetically, leading to more frequent and more energetic collisions. This increases the likelihood of successful collisions with enough energy to cause a reaction.
Concentration also plays a vital role in reaction rates. A more concentrated solution has more particles in the same volume, resulting in more frequent collisions between reactant particles. Similarly, increasing gas pressure means particles are closer together, also increasing collision frequency.
Try This: In the reaction between sodium thiosulphate and hydrochloric acid , you can observe the rate by timing how long it takes for the solution to turn cloudy yellow-green due to sulphur formation.
As a reaction progresses, you can measure how the volume or mass changes over time. The rate of these changes indicates the reaction rate - faster changes mean faster reactions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemical equilibrium is a fascinating state where forward and reverse reactions occur at exactly the same rate. It's like a chemical tug-of-war that results in a perfect balance.
There are different types of systems in chemistry: open systems release both mass and heat, closed systems release only heat, and isolated systems release nothing at all. Most equilibrium reactions we study occur in closed systems.
Le Chatelier's principle is crucial to understand: if a change is made to a system at equilibrium, the equilibrium position will shift to oppose that change. For example, if you add more reactants, the equilibrium shifts to use them up; if you remove products, it shifts to make more.
Visual Example: Hydrated copper sulphate (blue) and anhydrous copper sulphate (white) exist in equilibrium. Adding water shifts to the blue form; heating shifts to the white form.
In a dynamic equilibrium, the forward and reverse reactions continue to occur at equal rates, so there's no overall change in the amounts of reactants and products. This happens in reactions like CO₂(g) ⇌ CO₂(aq) and 2H₂ + O₂ ⇌ 2H₂O.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrolysis is a fascinating process that uses electrical current to drive chemical reactions that wouldn't happen spontaneously. It's how we extract reactive metals and produce many chemicals industrially.
At the anode (positive electrode), negatively charged ions are attracted and discharged. If halide ions (like chloride) are present, they're discharged. Otherwise, hydroxide ions (OH⁻) are discharged following the reaction: 4OH⁻ → 4e⁻ + O₂ + 2H₂O.
At the cathode (negative electrode), positively charged ions are attracted. The least reactive element will be discharged first. In solutions with hydrogen ions, you'll see: 2H⁺ + 2e⁻ → H₂.
Practical Tip: You can test for oxygen gas produced at the anode using damp blue litmus paper - it will bleach in the presence of oxygen.
To perform electrolysis, pour about 50cm³ of solution into a beaker, insert electrodes making sure they don't touch, connect to a 4V power supply, and run for about 5 minutes while making observations. Repeat with different solutions to compare results.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Transformers are clever devices that can change the voltage of alternating current electricity without wasting much energy. They're essential for our national electricity grid.
A transformer consists of two coils of wire (primary and secondary) wrapped around an iron core. When alternating current flows through the primary coil, it creates a changing magnetic field in the iron core. This changing field then induces a voltage in the secondary coil.
There are two main types of transformers: step-up transformers increase the voltage, while step-down transformers decrease it. The relationship between the voltages depends on the number of turns in each coil.
Remember: Transformers only work with alternating current (AC), never with direct current (DC), because you need a changing magnetic field to induce voltage in the secondary coil.
Transformers are based on electromagnetic induction, discovered by Michael Faraday. This principle is used throughout our electrical distribution system to efficiently deliver power to homes and businesses.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The National Grid is the network that distributes electricity across the country. It relies heavily on transformers to work efficiently.
Transformers in the National Grid follow a simple mathematical relationship: the ratio of voltages equals the inverse ratio of currents. If we increase the voltage across the secondary coil compared to the primary coil, we decrease the current in the secondary coil proportionally.
This relationship is crucial for efficient power transmission. By stepping up the voltage to very high levels for transmission over long distances, we reduce the current, which dramatically reduces energy losses due to heating in the wires.
Power Fact: The voltage in the National Grid's transmission lines can be as high as 400,000 volts, but this is stepped down in stages before reaching the 230V used in UK homes.
Near power stations, step-up transformers increase voltage for transmission. Then, step-down transformers at substations gradually reduce the voltage as electricity gets closer to homes and businesses, making it safe for domestic use.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
46
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the principles of osmosis through a detailed examination of a potato osmosis experiment. This resource covers key concepts such as hypertonic and hypotonic solutions, the methodology for conducting the experiment, and the expected outcomes based on varying sugar/salt concentrations. Ideal for AQA GCSE Science students preparing for practical assessments.
Explore key concepts in AQA Combined Science Biology Paper 1, including cellular structures, bioenergetics, human organization, and metabolism. This comprehensive summary covers essential topics such as photosynthesis, respiration, the heart and blood, and the human defense system, providing a solid foundation for exam preparation.
Explore essential GCSE AQA Biology practicals covering microscopy, osmosis, enzyme activity, and food tests. This summary includes detailed methods for experiments on photosynthesis, microorganism culturing, and the effects of pH on amylase. Ideal for students preparing for Biology Paper 1, this resource highlights key concepts and practical techniques.
Comprehensive study notes covering key concepts in cellular biology, human digestion, respiration, photosynthesis, and the circulatory system. This resource includes detailed explanations of cell structures, enzyme functions, nutrient absorption, and the impact of environmental factors on biological processes. Ideal for students preparing for Biology Paper 1 exams.
Explore the osmosis process through a hands-on potato experiment. This practical guide details the steps to investigate how different sucrose concentrations affect potato mass, including calculations for percentage change. Ideal for GCSE Biology students and anyone studying diffusion, osmosis, and tonicity concepts.
Explore essential biology practicals including enzyme activity, photosynthesis, microscopy, osmosis, and food tests. This comprehensive guide provides step-by-step procedures and key concepts for each experiment, perfect for students preparing for exams. Enhance your understanding of biological processes and laboratory techniques.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user