Chemical bonding involves different ways atoms join together to form... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Responding to change (a2 only)
Infection and response
Homeostasis and response
Energy transfers (a2 only)
Cell biology
Organisms respond to changes in their internal and external environments (a-level only)
Biological molecules
Organisation
Substance exchange
Bioenergetics
Genetic information & variation
Inheritance, variation and evolution
Genetics & ecosystems (a2 only)
Ecology
Cells
Show all topics
Britain & the wider world: 1745 -1901
1l the quest for political stability: germany, 1871-1991
The cold war
Inter-war germany
Medieval period: 1066 -1509
2d religious conflict and the church in england, c1529-c1570
2o democracy and nazism: germany, 1918-1945
1f industrialisation and the people: britain, c1783-1885
1c the tudors: england, 1485-1603
2m wars and welfare: britain in transition, 1906-1957
World war two & the holocaust
2n revolution and dictatorship: russia, 1917-1953
2s the making of modern britain, 1951-2007
World war one
Britain: 1509 -1745
Show all topics
1,180
•
27 Dec 2025
•
Chemical bonding involves different ways atoms join together to form... Show more










The reaction between ethene and bromine demonstrates important concepts about chemical energetics and bond breaking/forming. This addition reaction involves breaking the carbon-carbon double bond in ethene and the bromine-bromine bond, followed by forming new carbon-bromine single bonds.
The reaction profile ethene bromine energy diagram shows how the energy changes during the course of the reaction. The activation energy represents the energy barrier that must be overcome for the reaction to proceed. The overall energy change (ΔH) indicates that this is an exothermic reaction, releasing energy as new bonds form.
Highlight: In reactions of alkenes, the carbon-carbon double bond is particularly reactive. The π bond can break relatively easily, allowing addition reactions to occur with molecules like bromine and chlorine.
Bond energies provide crucial information about reaction energetics. Stronger bonds require more energy to break and release more energy when formed. The trend in bond strengths can be explained by atomic structure - atoms with more electron shells generally form weaker covalent bonds because their outer electrons are less strongly held.
Vocabulary: Bond enthalpy is the energy required to break one mole of bonds in gaseous molecules under standard conditions. This value helps predict the overall energy changes in chemical reactions.

This page contrasts the properties of ionic compounds like potassium sulfide with those of covalent compounds. It explains why ionic compounds have high boiling points and conduct electricity when molten or dissolved in water, relating these properties to the presence of free ions and strong ionic bonds.
The page then introduces a new set of questions about four substances (A, B, C, and D) with different structures and states of matter. Students are asked to identify which substance represents a gas, liquid, element, and ionic compound based on structural diagrams.
The final section begins a discussion on copper extraction through smelting, presenting a chemical equation for the reaction of copper(I) sulfide with oxygen to produce copper and sulfur dioxide.
Vocabulary: Smelting - A process of applying heat and a chemical reducing agent to ore to extract a base metal.
Example: The reaction Cu₂S(s) + O₂(g) → 2Cu(s) + SO₂(g) represents the smelting process for copper extraction.

Chemical bonds form the foundation of how atoms interact and combine to create different substances. The way electrons are arranged and shared between atoms determines the type of bonding and the resulting properties of compounds.
Ionic compounds form through electron transfer between metals and non-metals. When potassium reacts with sulfur to form potassium sulfide, the potassium atoms each lose one electron while the sulfur atom gains two electrons. This transfer of electrons creates oppositely charged ions that are held together by strong electrostatic forces. The resulting ionic compound has a crystalline structure with regular arrangements of positive potassium ions and negative sulfide ions.
Definition: The bond formed by transfer of electrons between atoms is called an ionic bond. This occurs when electrons completely transfer from one atom to another, creating oppositely charged ions.
When it comes to covalent bonding, atoms share rather than transfer electrons. In hydrogen sulfide (H2S), the sulfur atom shares electrons with two hydrogen atoms to form covalent bonds. The H2S molecular geometry is bent due to the presence of two lone pairs of electrons on the sulfur atom. This arrangement makes H2S polar, meaning it has an uneven distribution of electrical charge across the molecule.
Example: The H2S Lewis structure shows two single bonds between sulfur and hydrogen atoms, with two lone pairs of electrons on the sulfur atom. This gives H2S its characteristic bent shape and polar nature.

This section presents the structures of four different substances (A, B, C, and D) and asks students to identify their physical states and nature based on the diagrams.
Students are asked to determine which substance is a gas, a liquid, an element, and made of ions.
Highlight: The ability to interpret structural diagrams is crucial for understanding the properties and behavior of different substances.
The bonding in one of the substances (C) is examined in more detail.
Example: A diagram showing particles arranged in a regular lattice structure represents an ionic compound.

This page continues the exploration of substance properties, focusing on the relationship between structure and properties. It examines the properties of ionic compounds in more detail, explaining why they have high boiling points and conduct electricity when molten.
Highlight: The ability of ionic compounds to conduct electricity when molten is due to the presence of mobile ions.
The page introduces representations of different substances, labeled A, B, C, and D, and asks students to identify their states and compositions based on their structures. This exercise reinforces the connection between molecular structure and macroscopic properties.
Example: A substance represented by closely packed, ordered particles is likely to be a solid, while one with widely spaced particles is likely to be a gas.
The concept of elements versus compounds is revisited, with students asked to identify which of the represented substances is an element. This reinforces the fundamental distinction between elements and compounds in chemistry.
The page concludes by introducing a discussion on copper extraction, touching on the environmental implications of industrial processes. This brings in real-world applications of chemistry and introduces the concept of chemical reactions in industrial contexts.
Vocabulary: Smelting - The process of extracting metal from its ore by heating in a furnace.
This section bridges the gap between theoretical chemistry concepts and their practical applications in industry and environmental science.

This final section discusses the extraction and purification of copper from ores.
The smelting process for extracting copper from copper-rich ores is introduced, with a focus on one of the reactions involved:
Cu₂S(s) + O₂(g) → 2 Cu(s) + SO₂(g)
Highlight: Understanding the environmental impacts of industrial processes is an important aspect of modern chemistry.
Students are asked to explain the environmental problems that could arise if sulfur dioxide gas escaped into the atmosphere during this process.
The purification of the impure copper produced by smelting is also mentioned, though not elaborated upon in the given transcript.

The first page introduces questions about different substances and their structures. It covers ionic compounds, gases, liquids, and solid metals.
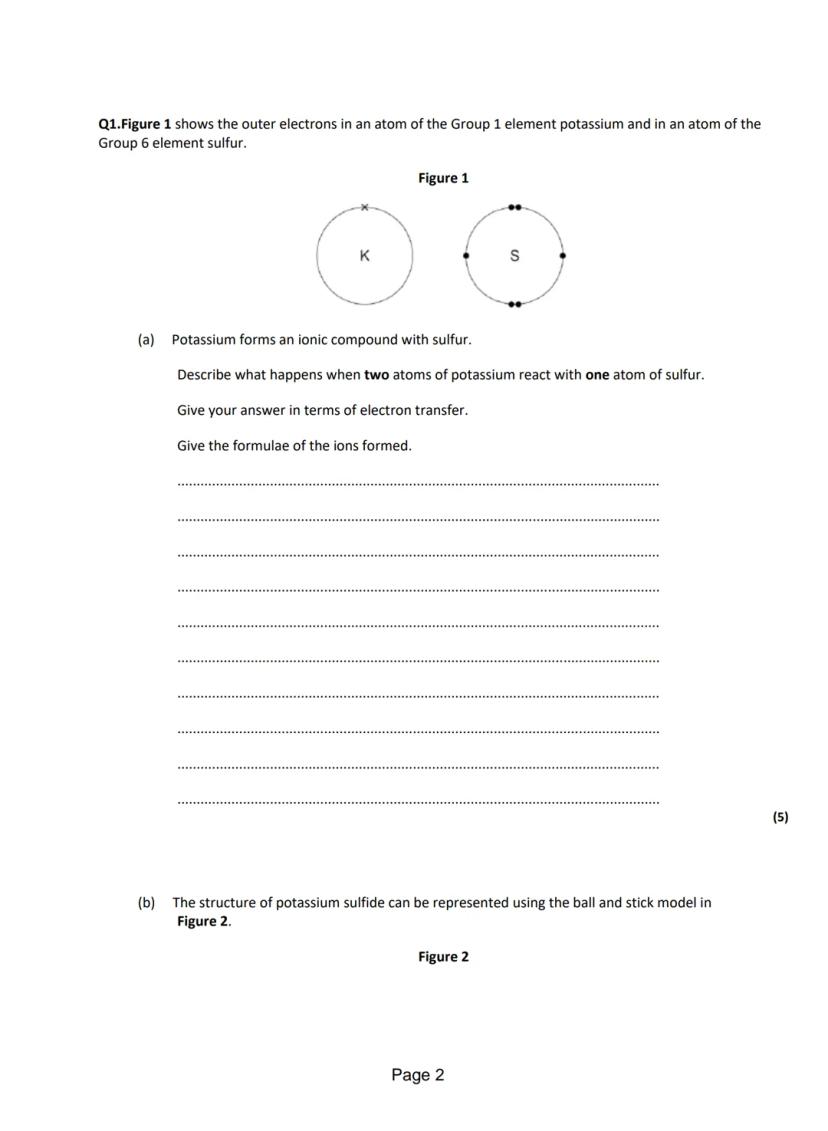
A key focus is on the reaction between potassium and sulfur atoms to form an ionic compound. This involves electron transfer between the Group 1 and Group 6 elements.
The page also discusses the reaction between ethene and bromine, including energy changes and covalent bonding.
Highlight: The reaction profile diagram illustrates important concepts like activation energy and enthalpy change for the ethene-bromine reaction.
Vocabulary: Covalent bond - A chemical bond formed by the sharing of electrons between atoms.



Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Chemical bonding involves different ways atoms join together to form compounds through the movement or sharing of electrons.
Ionic compounds form when electrons completely transfer between atoms, typically from metals to non-metals. During electron transfer, one atom loses electrons... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The reaction between ethene and bromine demonstrates important concepts about chemical energetics and bond breaking/forming. This addition reaction involves breaking the carbon-carbon double bond in ethene and the bromine-bromine bond, followed by forming new carbon-bromine single bonds.
The reaction profile ethene bromine energy diagram shows how the energy changes during the course of the reaction. The activation energy represents the energy barrier that must be overcome for the reaction to proceed. The overall energy change (ΔH) indicates that this is an exothermic reaction, releasing energy as new bonds form.
Highlight: In reactions of alkenes, the carbon-carbon double bond is particularly reactive. The π bond can break relatively easily, allowing addition reactions to occur with molecules like bromine and chlorine.
Bond energies provide crucial information about reaction energetics. Stronger bonds require more energy to break and release more energy when formed. The trend in bond strengths can be explained by atomic structure - atoms with more electron shells generally form weaker covalent bonds because their outer electrons are less strongly held.
Vocabulary: Bond enthalpy is the energy required to break one mole of bonds in gaseous molecules under standard conditions. This value helps predict the overall energy changes in chemical reactions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This page contrasts the properties of ionic compounds like potassium sulfide with those of covalent compounds. It explains why ionic compounds have high boiling points and conduct electricity when molten or dissolved in water, relating these properties to the presence of free ions and strong ionic bonds.
The page then introduces a new set of questions about four substances (A, B, C, and D) with different structures and states of matter. Students are asked to identify which substance represents a gas, liquid, element, and ionic compound based on structural diagrams.
The final section begins a discussion on copper extraction through smelting, presenting a chemical equation for the reaction of copper(I) sulfide with oxygen to produce copper and sulfur dioxide.
Vocabulary: Smelting - A process of applying heat and a chemical reducing agent to ore to extract a base metal.
Example: The reaction Cu₂S(s) + O₂(g) → 2Cu(s) + SO₂(g) represents the smelting process for copper extraction.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemical bonds form the foundation of how atoms interact and combine to create different substances. The way electrons are arranged and shared between atoms determines the type of bonding and the resulting properties of compounds.
Ionic compounds form through electron transfer between metals and non-metals. When potassium reacts with sulfur to form potassium sulfide, the potassium atoms each lose one electron while the sulfur atom gains two electrons. This transfer of electrons creates oppositely charged ions that are held together by strong electrostatic forces. The resulting ionic compound has a crystalline structure with regular arrangements of positive potassium ions and negative sulfide ions.
Definition: The bond formed by transfer of electrons between atoms is called an ionic bond. This occurs when electrons completely transfer from one atom to another, creating oppositely charged ions.
When it comes to covalent bonding, atoms share rather than transfer electrons. In hydrogen sulfide (H2S), the sulfur atom shares electrons with two hydrogen atoms to form covalent bonds. The H2S molecular geometry is bent due to the presence of two lone pairs of electrons on the sulfur atom. This arrangement makes H2S polar, meaning it has an uneven distribution of electrical charge across the molecule.
Example: The H2S Lewis structure shows two single bonds between sulfur and hydrogen atoms, with two lone pairs of electrons on the sulfur atom. This gives H2S its characteristic bent shape and polar nature.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This section presents the structures of four different substances (A, B, C, and D) and asks students to identify their physical states and nature based on the diagrams.
Students are asked to determine which substance is a gas, a liquid, an element, and made of ions.
Highlight: The ability to interpret structural diagrams is crucial for understanding the properties and behavior of different substances.
The bonding in one of the substances (C) is examined in more detail.
Example: A diagram showing particles arranged in a regular lattice structure represents an ionic compound.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This page continues the exploration of substance properties, focusing on the relationship between structure and properties. It examines the properties of ionic compounds in more detail, explaining why they have high boiling points and conduct electricity when molten.
Highlight: The ability of ionic compounds to conduct electricity when molten is due to the presence of mobile ions.
The page introduces representations of different substances, labeled A, B, C, and D, and asks students to identify their states and compositions based on their structures. This exercise reinforces the connection between molecular structure and macroscopic properties.
Example: A substance represented by closely packed, ordered particles is likely to be a solid, while one with widely spaced particles is likely to be a gas.
The concept of elements versus compounds is revisited, with students asked to identify which of the represented substances is an element. This reinforces the fundamental distinction between elements and compounds in chemistry.
The page concludes by introducing a discussion on copper extraction, touching on the environmental implications of industrial processes. This brings in real-world applications of chemistry and introduces the concept of chemical reactions in industrial contexts.
Vocabulary: Smelting - The process of extracting metal from its ore by heating in a furnace.
This section bridges the gap between theoretical chemistry concepts and their practical applications in industry and environmental science.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This final section discusses the extraction and purification of copper from ores.
The smelting process for extracting copper from copper-rich ores is introduced, with a focus on one of the reactions involved:
Cu₂S(s) + O₂(g) → 2 Cu(s) + SO₂(g)
Highlight: Understanding the environmental impacts of industrial processes is an important aspect of modern chemistry.
Students are asked to explain the environmental problems that could arise if sulfur dioxide gas escaped into the atmosphere during this process.
The purification of the impure copper produced by smelting is also mentioned, though not elaborated upon in the given transcript.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The first page introduces questions about different substances and their structures. It covers ionic compounds, gases, liquids, and solid metals.
A key focus is on the reaction between potassium and sulfur atoms to form an ionic compound. This involves electron transfer between the Group 1 and Group 6 elements.
The page also discusses the reaction between ethene and bromine, including energy changes and covalent bonding.
Highlight: The reaction profile diagram illustrates important concepts like activation energy and enthalpy change for the ethene-bromine reaction.
Vocabulary: Covalent bond - A chemical bond formed by the sharing of electrons between atoms.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
31
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore key concepts in atomic structure, the periodic table, and the properties of matter. This comprehensive revision note covers atomic models, chemical bonding (ionic, covalent, and metallic), and the characteristics of elements in groups such as alkali metals and noble gases. Ideal for GCSE students preparing for exams.
Explore essential GCSE Chemistry concepts including atomic structure, chemical bonding, redox reactions, and electrolysis. This comprehensive mind map is designed for Grade 9 students preparing for the OCR exam board, covering key topics such as ionic and covalent compounds, oxidation, and neutralization reactions.
Explore the fundamentals of ion formation in chemistry, focusing on the role of protons, electrons, and neutrons. This summary covers key concepts such as isotopes, electron configuration in the periodic table, and the behavior of different groups in forming ions. Ideal for Year 9 students studying ionic bonding and chemical ions.
Explore the fundamentals of covalent bonding, including single, double, and triple bonds, properties of small covalent molecules, and limitations of dot and cross diagrams. This summary covers key concepts such as shared electron pairs, intermolecular forces, and energy level diagrams, making it essential for understanding chemical bonding in non-metal elements.
Explore the fundamental concepts of elements and atoms, including definitions, properties, and common chemical symbols. This summary covers key terms such as chemical, molecule, formula, polymer, and compound, essential for Year 8 science students.
Explore the essential concepts of atomic structure and the periodic table for AQA GCSE Combined Science. This comprehensive summary covers elements, compounds, mixtures, separation techniques, and the development of the periodic table, including key groups like alkali metals and noble gases. Perfect for exam preparation and understanding fundamental chemistry principles.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user