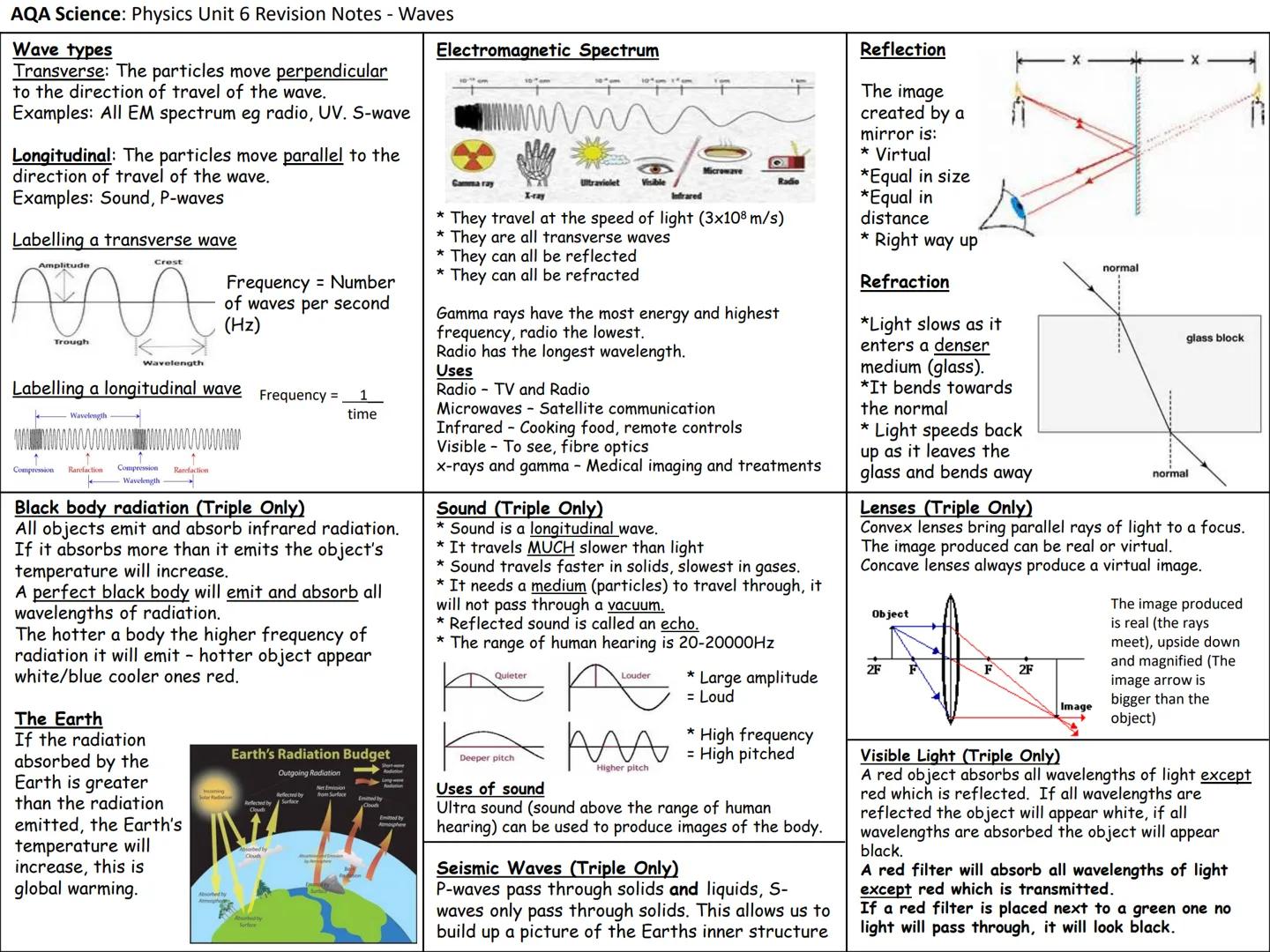
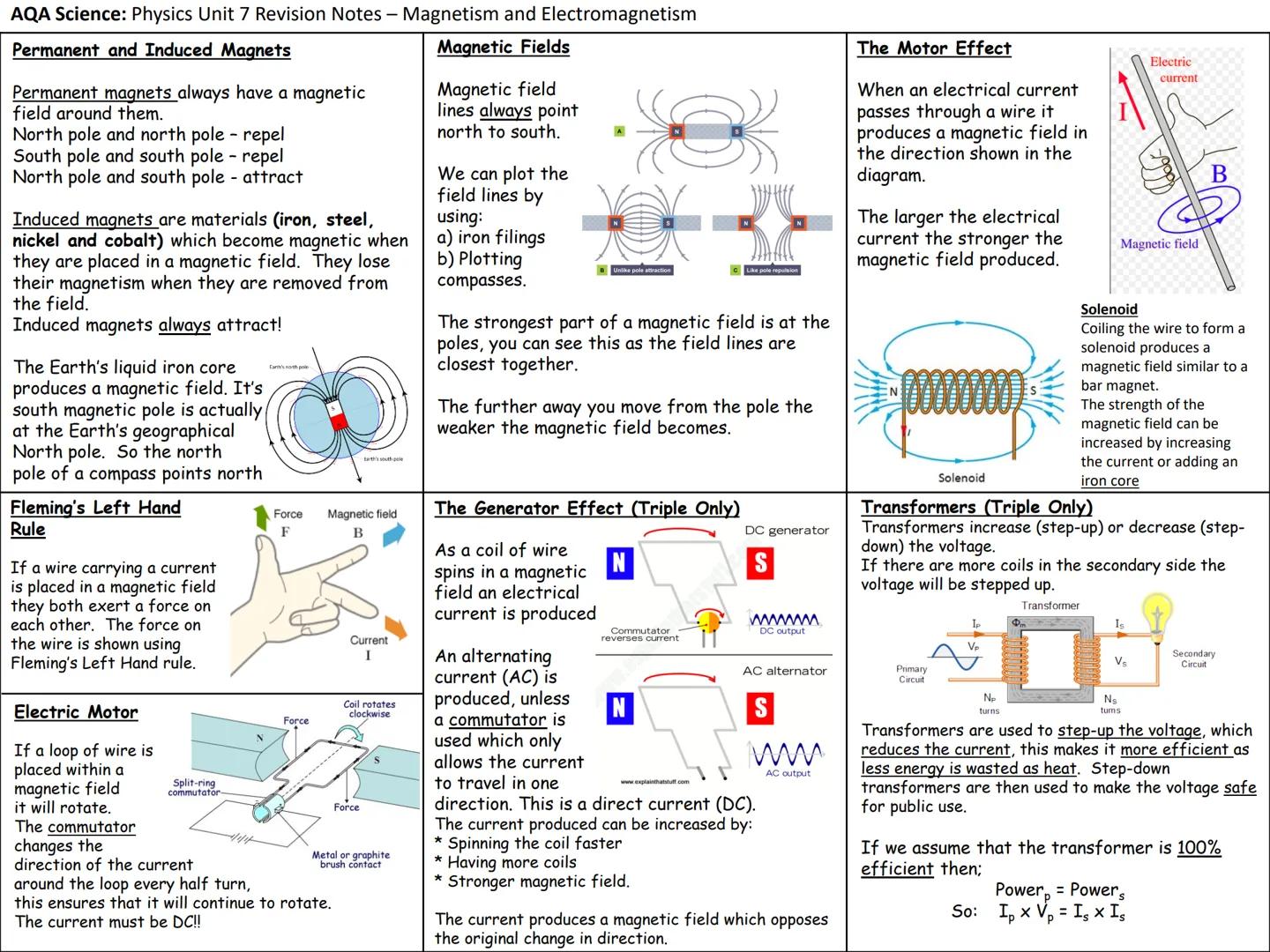
Unit 4: Radioactivity - Atomic Structure and Decay
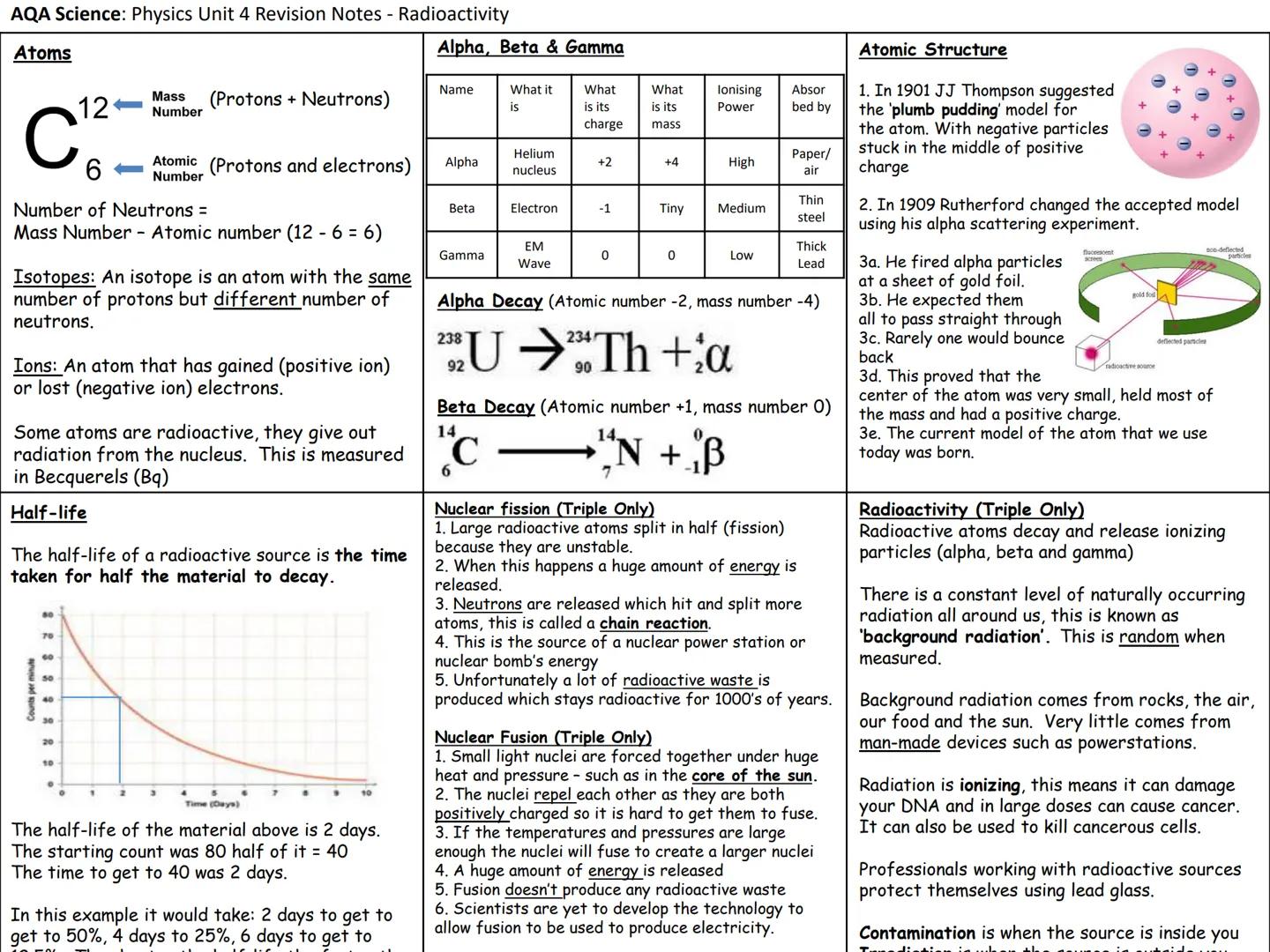
Atoms contain protons and neutrons in the nucleus, with electrons orbiting outside. The mass number counts protons plus neutrons, while atomic number counts just protons. Isotopes have identical proton numbers but different neutron counts.
Radioactive decay produces three radiation types. Alpha particles are helium nuclei charge+2,mass4, beta particles are electrons charge−1,tinymass, and gamma rays are electromagnetic waves (no charge or mass). Paper stops alpha, thin steel stops beta, thick lead stops gamma.
Half-life measures how long half the radioactive material takes to decay. If you start with 80 counts and drop to 40 counts in 2 days, the half-life is 2 days. After another 2 days, you'll have 20 counts remaining.
Nuclear reactions release enormous energy. Fission splits large unstable nuclei, creating chain reactions used in power stations. Fusion forces small nuclei together under extreme conditions like inside stars, producing even more energy with less radioactive waste.
Remember: Alpha decay reduces atomic number by 2 and mass by 4. Beta decay increases atomic number by 1 with no mass change.