Ever wondered what's inside an atom or why some materials... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
43
•
14 Feb 2026
•
Princess
@rincess_djayboeylvow
Ever wondered what's inside an atom or why some materials... Show more




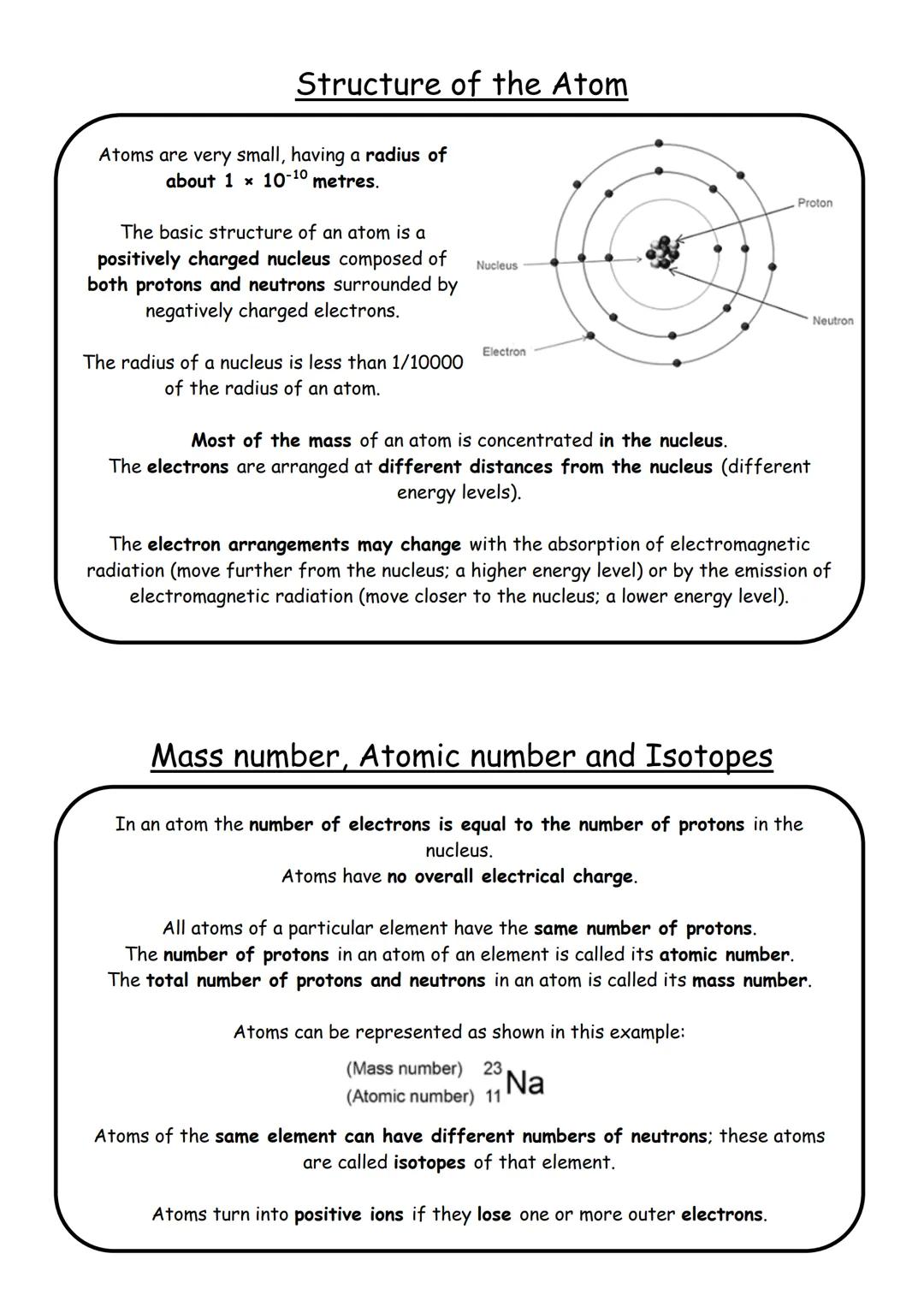
Think of an atom like a tiny solar system that's incredibly small - about 1 x 10⁻¹⁰ metres in radius! At the centre sits the nucleus, which contains positively charged protons and neutral neutrons. Whizzing around this nucleus are negatively charged electrons.
Here's the mental picture you need: the nucleus is absolutely tiny compared to the whole atom - less than 1/10,000 of the atom's radius. Yet nearly all the atom's mass is squashed into this minuscule centre. It's like having a marble in the middle of a football stadium!
Electrons don't just float anywhere - they're arranged at specific distances from the nucleus called energy levels. They can jump between these levels by absorbing or releasing electromagnetic radiation. When they absorb energy, they move further out; when they release energy, they move closer in.
Quick Tip: Remember that atoms are electrically neutral - the number of positive protons always equals the number of negative electrons in a normal atom!
The atomic number tells you how many protons an element has, while the mass number is the total of protons plus neutrons. Isotopes are atoms of the same element with different numbers of neutrons - same atomic number, different mass number.

Scientists didn't always know what atoms looked like - our understanding has completely transformed over time! Originally, people thought atoms were just tiny solid spheres that couldn't be broken apart. Pretty simple, right?
Everything changed when scientists discovered electrons. This led to the plum pudding model - imagine a ball of positive charge with negative electrons stuck in it like raisins in a pudding. Sounds tasty, but it was wrong!
The game-changer came with the alpha particle scattering experiment. Scientists fired tiny particles at atoms and watched what happened. Most particles went straight through, but some bounced back dramatically. This proved that atoms have a tiny, dense, positively charged centre - the nucleus.
Remember This: Niels Bohr then improved the model by suggesting electrons orbit the nucleus at fixed distances, like planets around the sun!
Later discoveries revealed that the nucleus itself contains protons and neutrons. James Chadwick discovered neutrons about 20 years after the nuclear model was accepted. Science builds on previous discoveries - each new piece of evidence helps us understand atoms better.
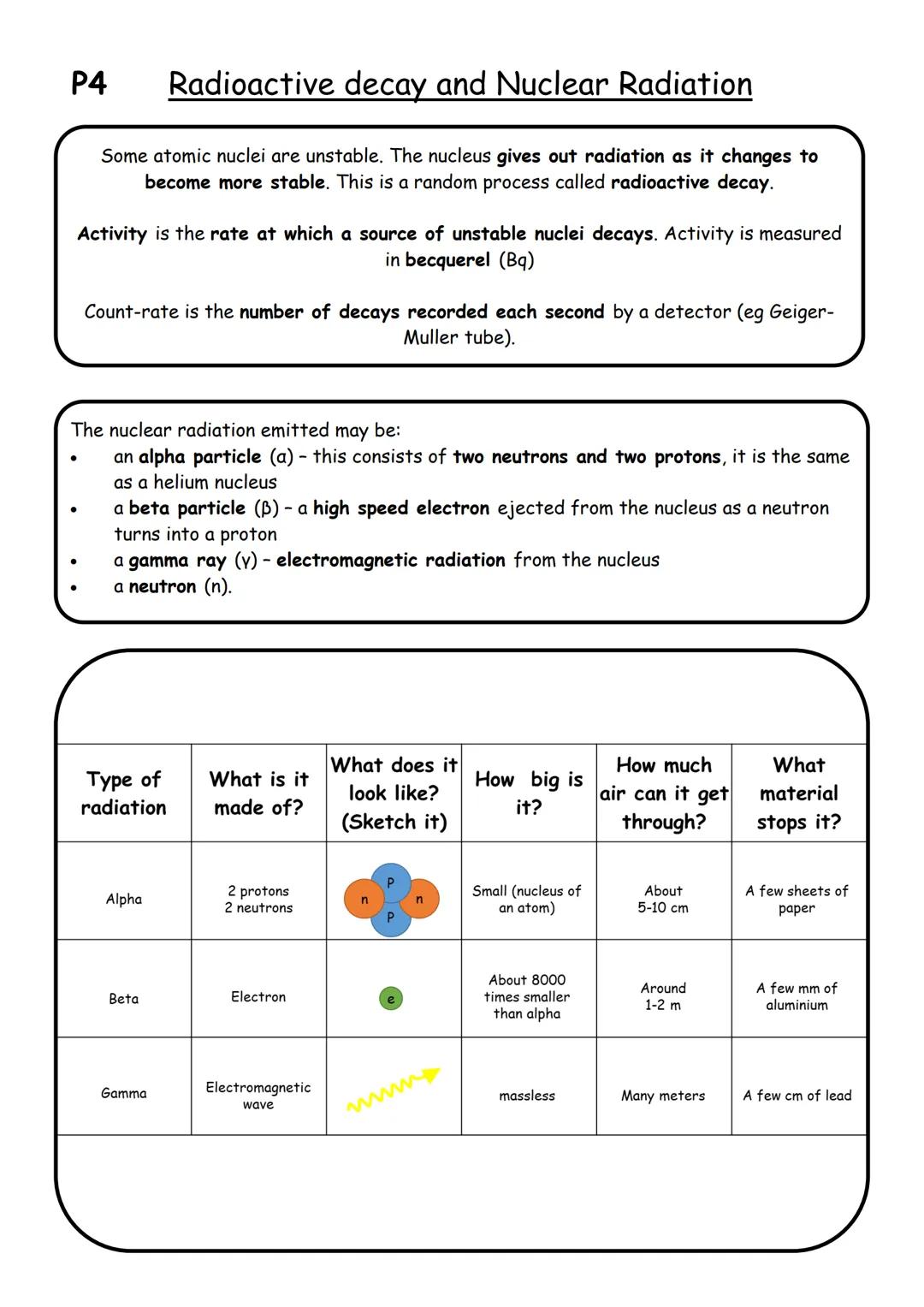
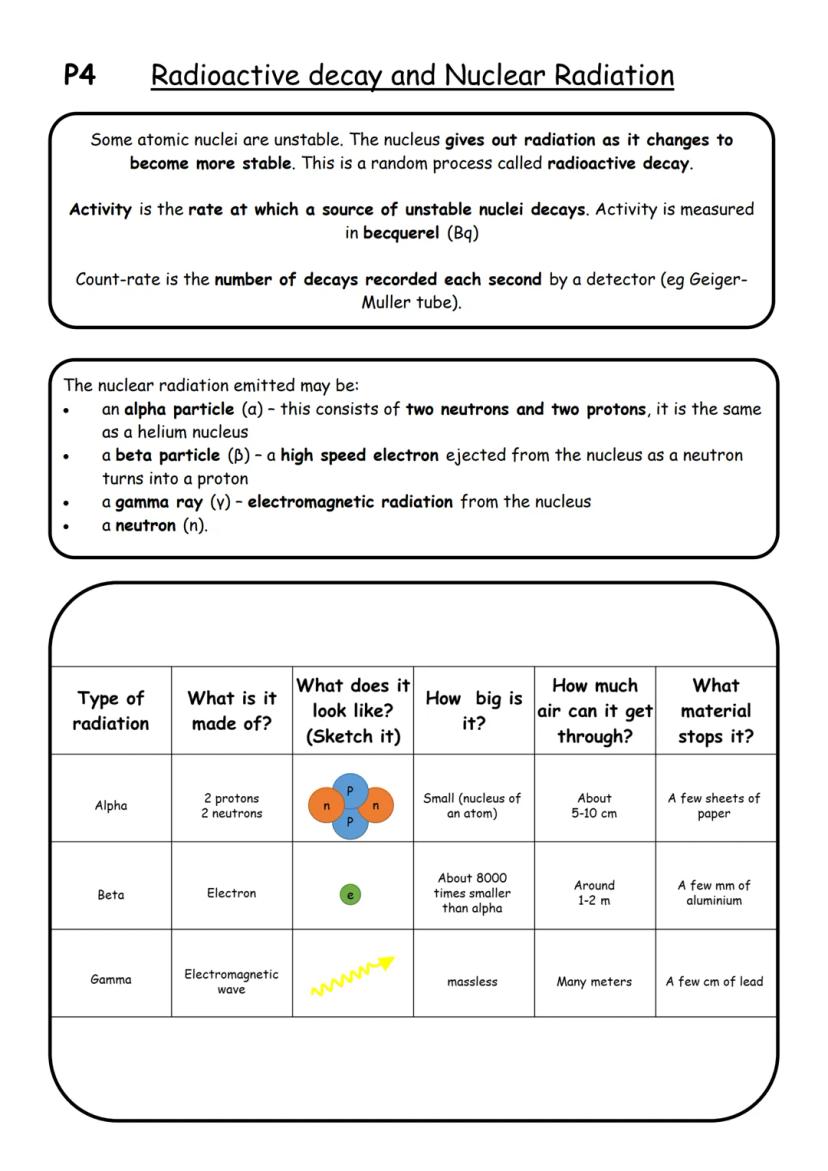
Some atomic nuclei are like unstable buildings - they're going to collapse eventually! Radioactive decay happens when unstable nuclei break down randomly to become more stable, giving off radiation in the process.
Activity measures how fast a radioactive source decays (measured in becquerel or Bq), while count-rate is what a detector actually records each second. Think of it like measuring rainfall - activity is how much rain falls, count-rate is what your rain gauge catches.
There are four main types of nuclear radiation you need to know. Alpha particles are chunky - they're helium nuclei with 2 protons and 2 neutrons. Beta particles are speedy electrons shot out when neutrons become protons. Gamma rays are pure electromagnetic energy, and neutrons are, well, neutrons!
Memory Trick: Think "Paper, Aluminium, Lead" - that's what stops alpha, beta, and gamma radiation respectively!
Each type has different penetrating power. Alpha particles barely make it through air , beta particles can travel further , and gamma rays can zip through metres of material before being stopped by lead.
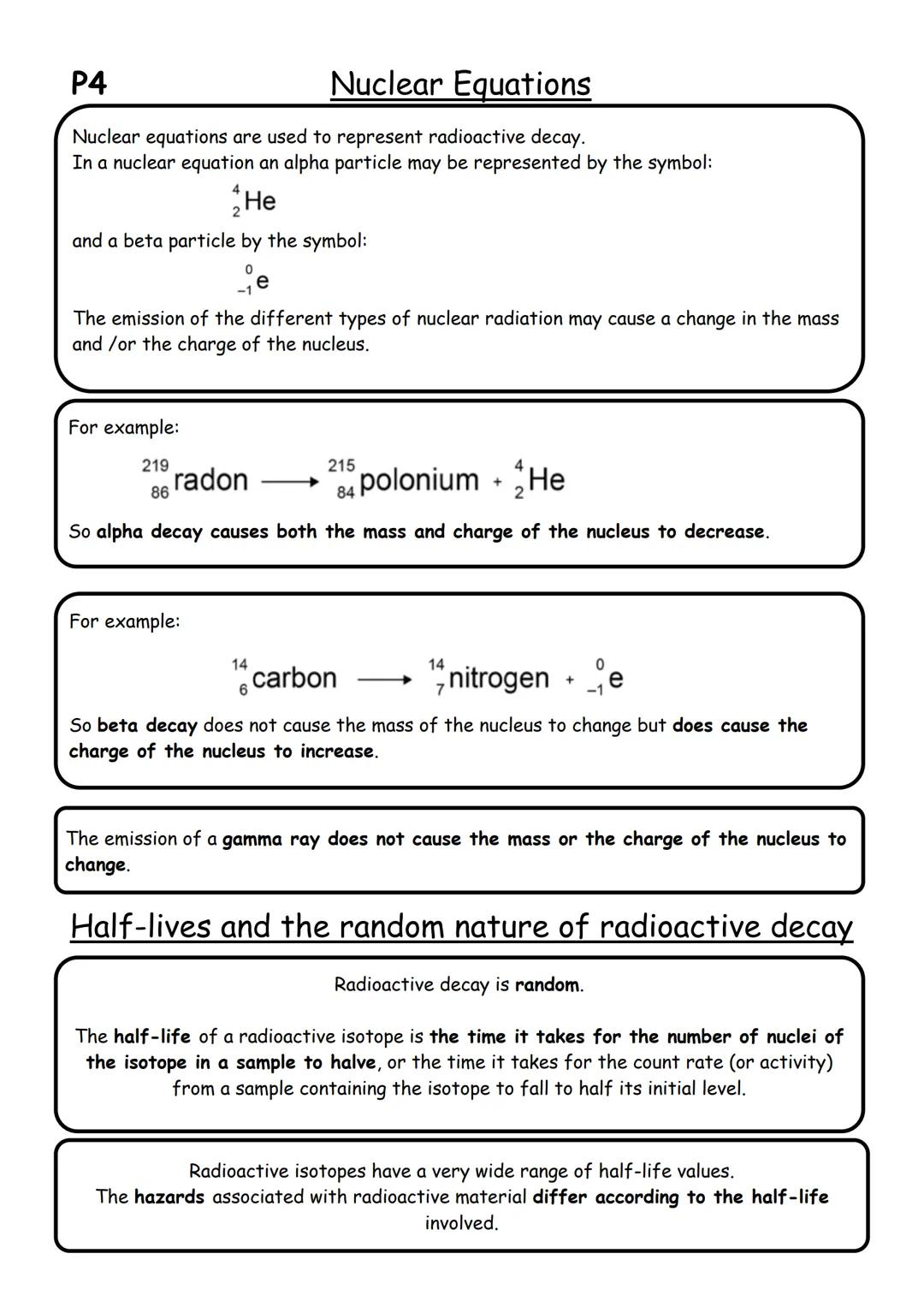
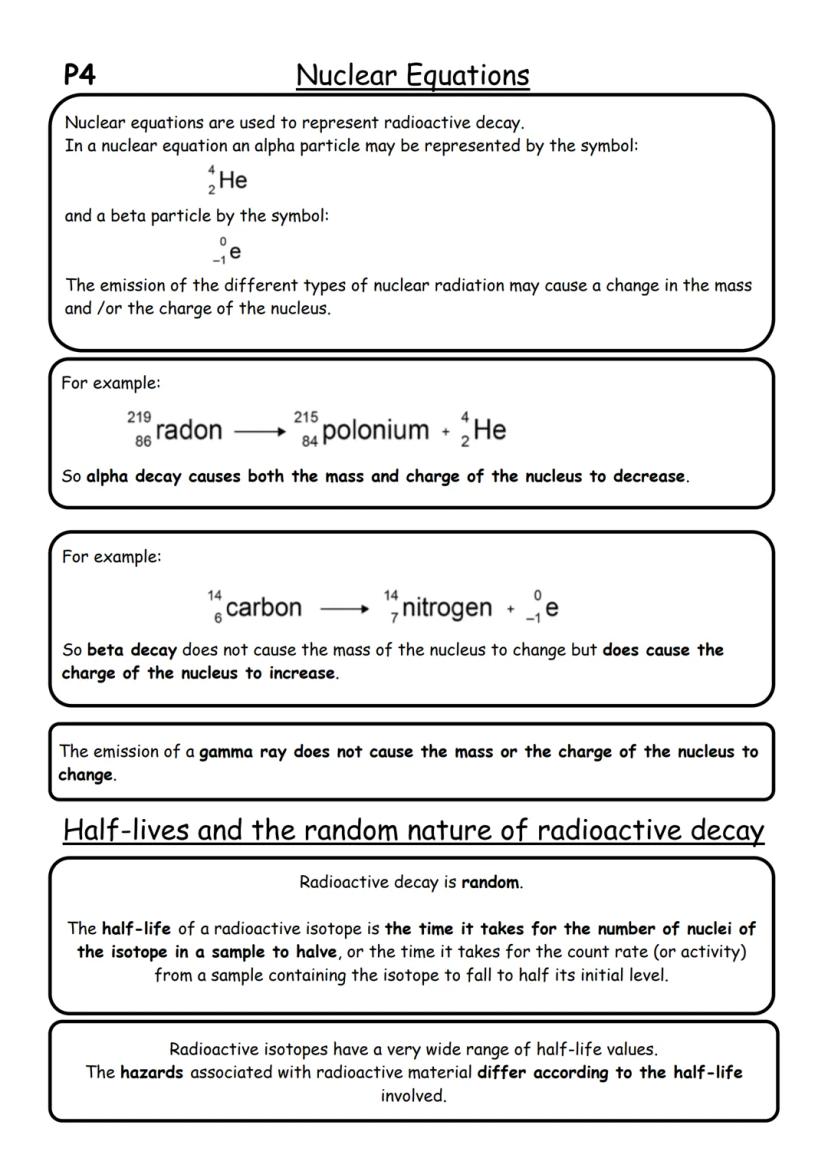
Nuclear equations are like maths for atoms - they show exactly what happens during radioactive decay. When writing these equations, you need to balance both mass numbers and atomic numbers, just like balancing a normal equation.
In alpha decay, the nucleus loses 2 protons and 2 neutrons, so both mass and charge decrease. For example, radon-219 becomes polonium-215 plus an alpha particle. In beta decay, a neutron becomes a proton, so the charge increases but mass stays the same.
Radioactive decay is completely random - you can't predict when any individual nucleus will decay. However, half-life gives us a useful pattern. It's the time needed for half the nuclei in a sample to decay.
Key Point: Half-life can range from fractions of seconds to millions of years - this affects how dangerous different radioactive materials are!
Here's how half-life works: if you start with 1000 radioactive nuclei and the half-life is 10 years, after 10 years you'll have 500 left. After another 10 years, you'll have 250, then 125, and so on. The count-rate follows the same pattern.

Radioactive contamination is when radioactive stuff gets where it shouldn't be - think of it like radioactive dust settling on objects. The contaminated object becomes dangerous because it's now giving off radiation as the radioactive atoms decay.
Irradiation is different - it's like shining a radioactive torch on something. The object gets exposed to radiation but doesn't become radioactive itself. Think about having an X-ray - you don't become radioactive afterwards!
Safety is crucial when dealing with radioactive materials. Scientists use protective equipment, work behind shields, and carefully control exposure time and distance. The type of radiation matters hugely for determining the level of danger.
Important: Background radiation is everywhere around us naturally - from rocks, cosmic rays from space, and some human-made sources like nuclear weapon testing fallout.
Radiation dose is measured in sieverts (Sv), with 1000 millisieverts (mSv) equalling 1 sievert. Your location and job can affect how much background radiation you're exposed to. People living in granite areas or working in certain industries might experience higher levels.

Nuclear radiation isn't just dangerous - it's incredibly useful in medicine! Doctors use radioactive materials to peek inside your body and see how organs are working. They inject tiny amounts of radioactive substances that travel through your bloodstream to specific organs.
The radiation coming from these organs gets measured and processed by computers to create detailed images. It's like having a GPS tracker that shows exactly how your kidneys or thyroid are functioning. Pretty clever, right?
Gamma radiation can also target and destroy cancer cells. Doctors carefully aim gamma rays at tumours while protecting healthy tissue around them. It's precision medicine at its finest.
Safety First: Even medical radiation carries risks - too much can cause cells to become cancerous or malfunction!
The key is responsible use. Medical professionals carefully calculate doses to get the benefits while minimising risks. They follow strict safety protocols and only use radiation when the benefits clearly outweigh the dangers. Understanding these risks helps patients make informed decisions about their treatment.
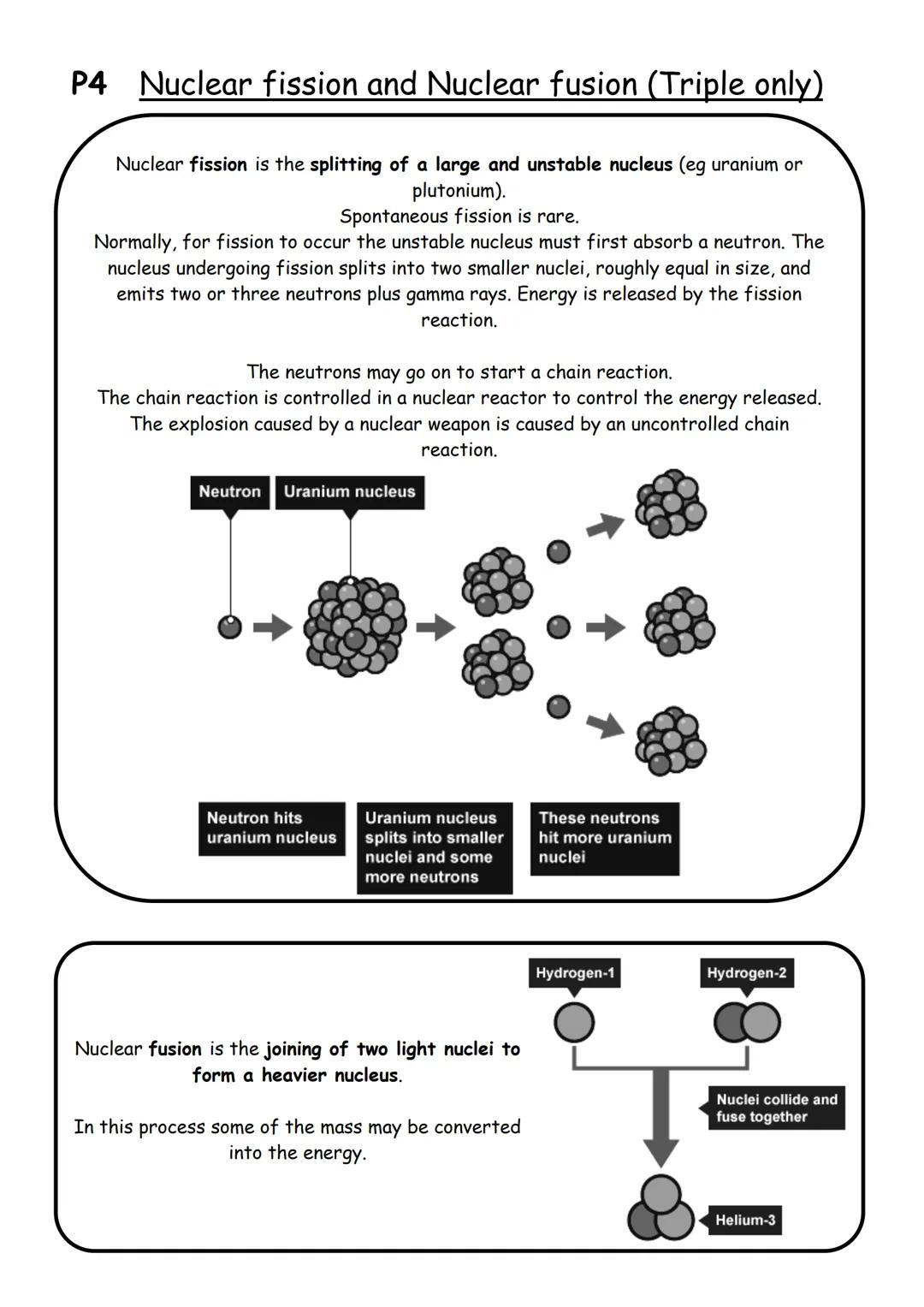
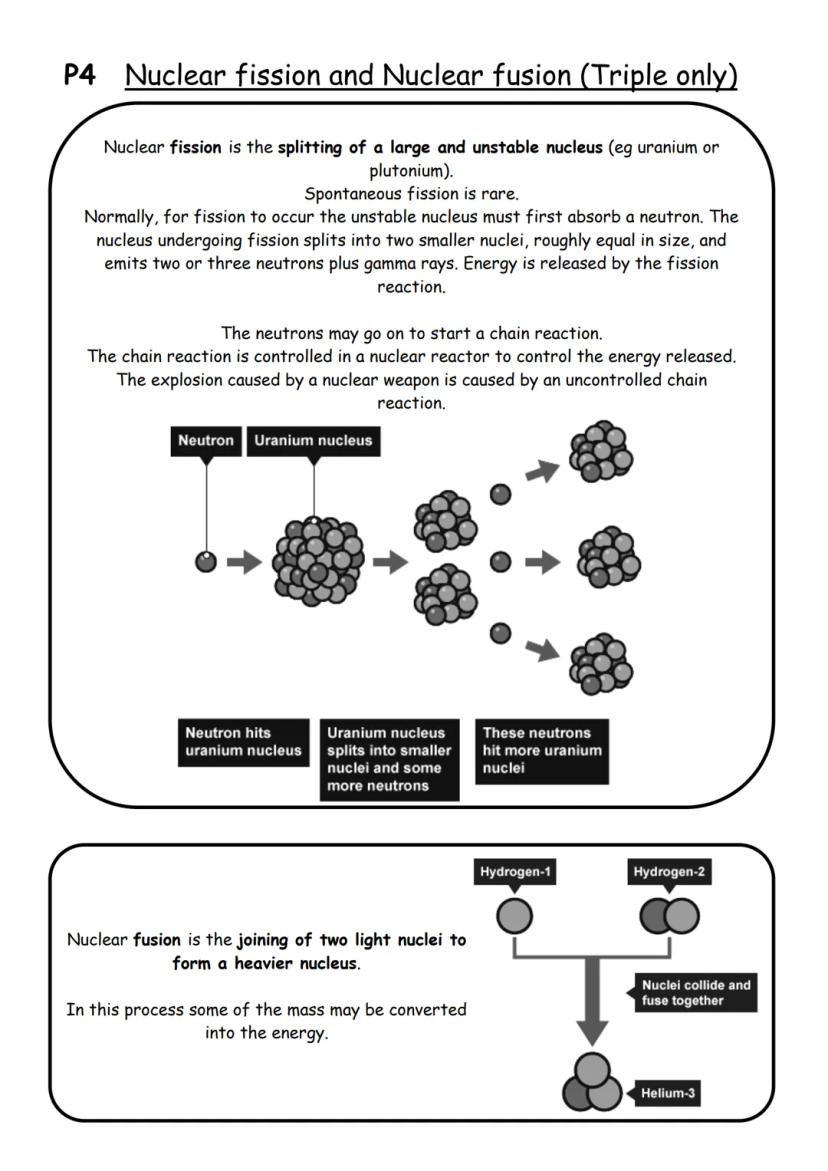
Nuclear fission is like cracking a massive egg - you split a large, unstable nucleus (like uranium or plutonium) into two smaller pieces. Most of the time, you need to fire a neutron at the nucleus to make this happen.
When fission occurs, the nucleus breaks into two roughly equal smaller nuclei and shoots out 2-3 more neutrons plus gamma rays. Here's the exciting bit - this releases enormous amounts of energy! Those extra neutrons can hit other nuclei, causing them to split too, creating a chain reaction.
In nuclear reactors, this chain reaction is carefully controlled to generate electricity. In nuclear weapons, it's deliberately uncontrolled, causing massive explosions. The difference between useful energy and devastating destruction is all about control.
Amazing Fact: In nuclear fusion, light nuclei join together to form heavier ones - this is what powers the Sun!
Nuclear fusion is the opposite process - instead of splitting heavy nuclei apart, you smash light nuclei (like hydrogen) together to make heavier ones (like helium). This also releases incredible amounts of energy and converts some mass into energy. Scientists are working to harness fusion for clean electricity generation.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Princess
@rincess_djayboeylvow
Ever wondered what's inside an atom or why some materials are radioactive? Understanding atomic structure and nuclear radiation is crucial for grasping how our world works at the smallest level. From medical treatments to nuclear power, these concepts shape modern... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of an atom like a tiny solar system that's incredibly small - about 1 x 10⁻¹⁰ metres in radius! At the centre sits the nucleus, which contains positively charged protons and neutral neutrons. Whizzing around this nucleus are negatively charged electrons.
Here's the mental picture you need: the nucleus is absolutely tiny compared to the whole atom - less than 1/10,000 of the atom's radius. Yet nearly all the atom's mass is squashed into this minuscule centre. It's like having a marble in the middle of a football stadium!
Electrons don't just float anywhere - they're arranged at specific distances from the nucleus called energy levels. They can jump between these levels by absorbing or releasing electromagnetic radiation. When they absorb energy, they move further out; when they release energy, they move closer in.
Quick Tip: Remember that atoms are electrically neutral - the number of positive protons always equals the number of negative electrons in a normal atom!
The atomic number tells you how many protons an element has, while the mass number is the total of protons plus neutrons. Isotopes are atoms of the same element with different numbers of neutrons - same atomic number, different mass number.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Scientists didn't always know what atoms looked like - our understanding has completely transformed over time! Originally, people thought atoms were just tiny solid spheres that couldn't be broken apart. Pretty simple, right?
Everything changed when scientists discovered electrons. This led to the plum pudding model - imagine a ball of positive charge with negative electrons stuck in it like raisins in a pudding. Sounds tasty, but it was wrong!
The game-changer came with the alpha particle scattering experiment. Scientists fired tiny particles at atoms and watched what happened. Most particles went straight through, but some bounced back dramatically. This proved that atoms have a tiny, dense, positively charged centre - the nucleus.
Remember This: Niels Bohr then improved the model by suggesting electrons orbit the nucleus at fixed distances, like planets around the sun!
Later discoveries revealed that the nucleus itself contains protons and neutrons. James Chadwick discovered neutrons about 20 years after the nuclear model was accepted. Science builds on previous discoveries - each new piece of evidence helps us understand atoms better.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Some atomic nuclei are like unstable buildings - they're going to collapse eventually! Radioactive decay happens when unstable nuclei break down randomly to become more stable, giving off radiation in the process.
Activity measures how fast a radioactive source decays (measured in becquerel or Bq), while count-rate is what a detector actually records each second. Think of it like measuring rainfall - activity is how much rain falls, count-rate is what your rain gauge catches.
There are four main types of nuclear radiation you need to know. Alpha particles are chunky - they're helium nuclei with 2 protons and 2 neutrons. Beta particles are speedy electrons shot out when neutrons become protons. Gamma rays are pure electromagnetic energy, and neutrons are, well, neutrons!
Memory Trick: Think "Paper, Aluminium, Lead" - that's what stops alpha, beta, and gamma radiation respectively!
Each type has different penetrating power. Alpha particles barely make it through air , beta particles can travel further , and gamma rays can zip through metres of material before being stopped by lead.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Nuclear equations are like maths for atoms - they show exactly what happens during radioactive decay. When writing these equations, you need to balance both mass numbers and atomic numbers, just like balancing a normal equation.
In alpha decay, the nucleus loses 2 protons and 2 neutrons, so both mass and charge decrease. For example, radon-219 becomes polonium-215 plus an alpha particle. In beta decay, a neutron becomes a proton, so the charge increases but mass stays the same.
Radioactive decay is completely random - you can't predict when any individual nucleus will decay. However, half-life gives us a useful pattern. It's the time needed for half the nuclei in a sample to decay.
Key Point: Half-life can range from fractions of seconds to millions of years - this affects how dangerous different radioactive materials are!
Here's how half-life works: if you start with 1000 radioactive nuclei and the half-life is 10 years, after 10 years you'll have 500 left. After another 10 years, you'll have 250, then 125, and so on. The count-rate follows the same pattern.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Radioactive contamination is when radioactive stuff gets where it shouldn't be - think of it like radioactive dust settling on objects. The contaminated object becomes dangerous because it's now giving off radiation as the radioactive atoms decay.
Irradiation is different - it's like shining a radioactive torch on something. The object gets exposed to radiation but doesn't become radioactive itself. Think about having an X-ray - you don't become radioactive afterwards!
Safety is crucial when dealing with radioactive materials. Scientists use protective equipment, work behind shields, and carefully control exposure time and distance. The type of radiation matters hugely for determining the level of danger.
Important: Background radiation is everywhere around us naturally - from rocks, cosmic rays from space, and some human-made sources like nuclear weapon testing fallout.
Radiation dose is measured in sieverts (Sv), with 1000 millisieverts (mSv) equalling 1 sievert. Your location and job can affect how much background radiation you're exposed to. People living in granite areas or working in certain industries might experience higher levels.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Nuclear radiation isn't just dangerous - it's incredibly useful in medicine! Doctors use radioactive materials to peek inside your body and see how organs are working. They inject tiny amounts of radioactive substances that travel through your bloodstream to specific organs.
The radiation coming from these organs gets measured and processed by computers to create detailed images. It's like having a GPS tracker that shows exactly how your kidneys or thyroid are functioning. Pretty clever, right?
Gamma radiation can also target and destroy cancer cells. Doctors carefully aim gamma rays at tumours while protecting healthy tissue around them. It's precision medicine at its finest.
Safety First: Even medical radiation carries risks - too much can cause cells to become cancerous or malfunction!
The key is responsible use. Medical professionals carefully calculate doses to get the benefits while minimising risks. They follow strict safety protocols and only use radiation when the benefits clearly outweigh the dangers. Understanding these risks helps patients make informed decisions about their treatment.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Nuclear fission is like cracking a massive egg - you split a large, unstable nucleus (like uranium or plutonium) into two smaller pieces. Most of the time, you need to fire a neutron at the nucleus to make this happen.
When fission occurs, the nucleus breaks into two roughly equal smaller nuclei and shoots out 2-3 more neutrons plus gamma rays. Here's the exciting bit - this releases enormous amounts of energy! Those extra neutrons can hit other nuclei, causing them to split too, creating a chain reaction.
In nuclear reactors, this chain reaction is carefully controlled to generate electricity. In nuclear weapons, it's deliberately uncontrolled, causing massive explosions. The difference between useful energy and devastating destruction is all about control.
Amazing Fact: In nuclear fusion, light nuclei join together to form heavier ones - this is what powers the Sun!
Nuclear fusion is the opposite process - instead of splitting heavy nuclei apart, you smash light nuclei (like hydrogen) together to make heavier ones (like helium). This also releases incredible amounts of energy and converts some mass into energy. Scientists are working to harness fusion for clean electricity generation.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
1
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user