Atomic physics covers the fundamental building blocks of matter and... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
88
•
6 Feb 2026
•
thressi kutty
@thressikutty_naiu
Atomic physics covers the fundamental building blocks of matter and... Show more
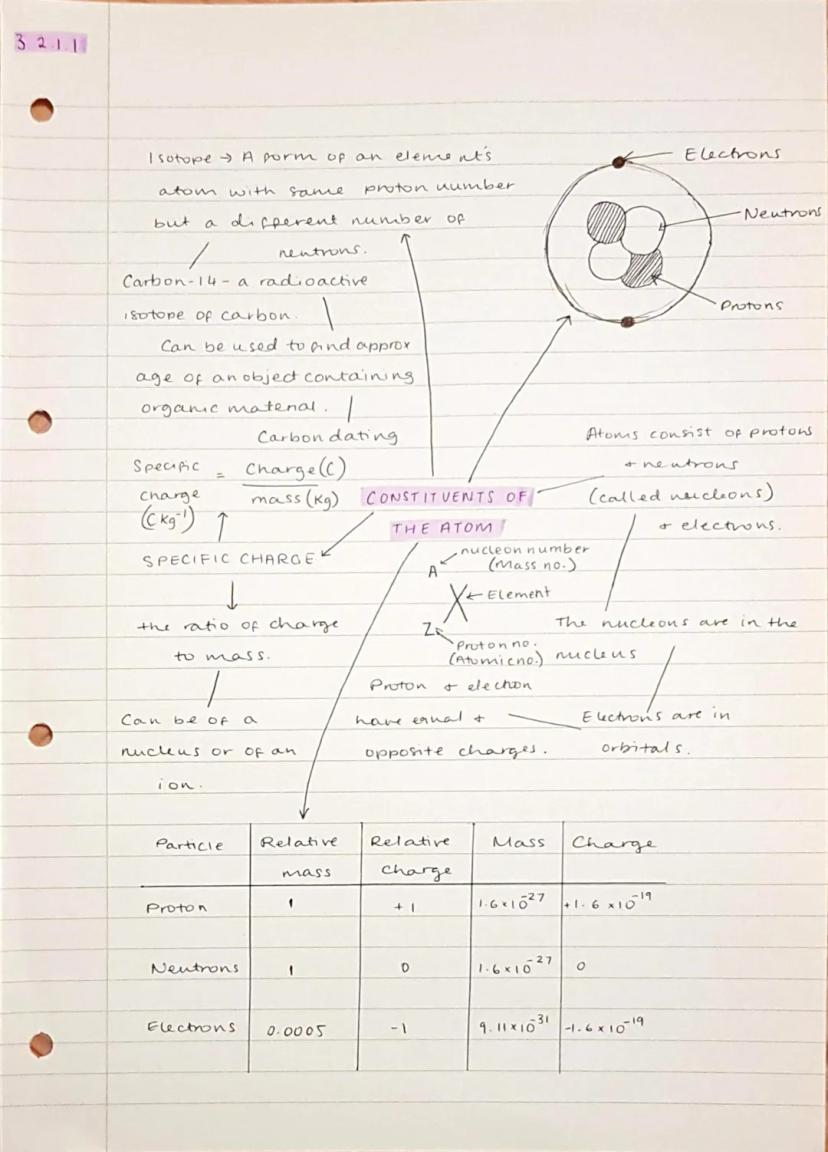



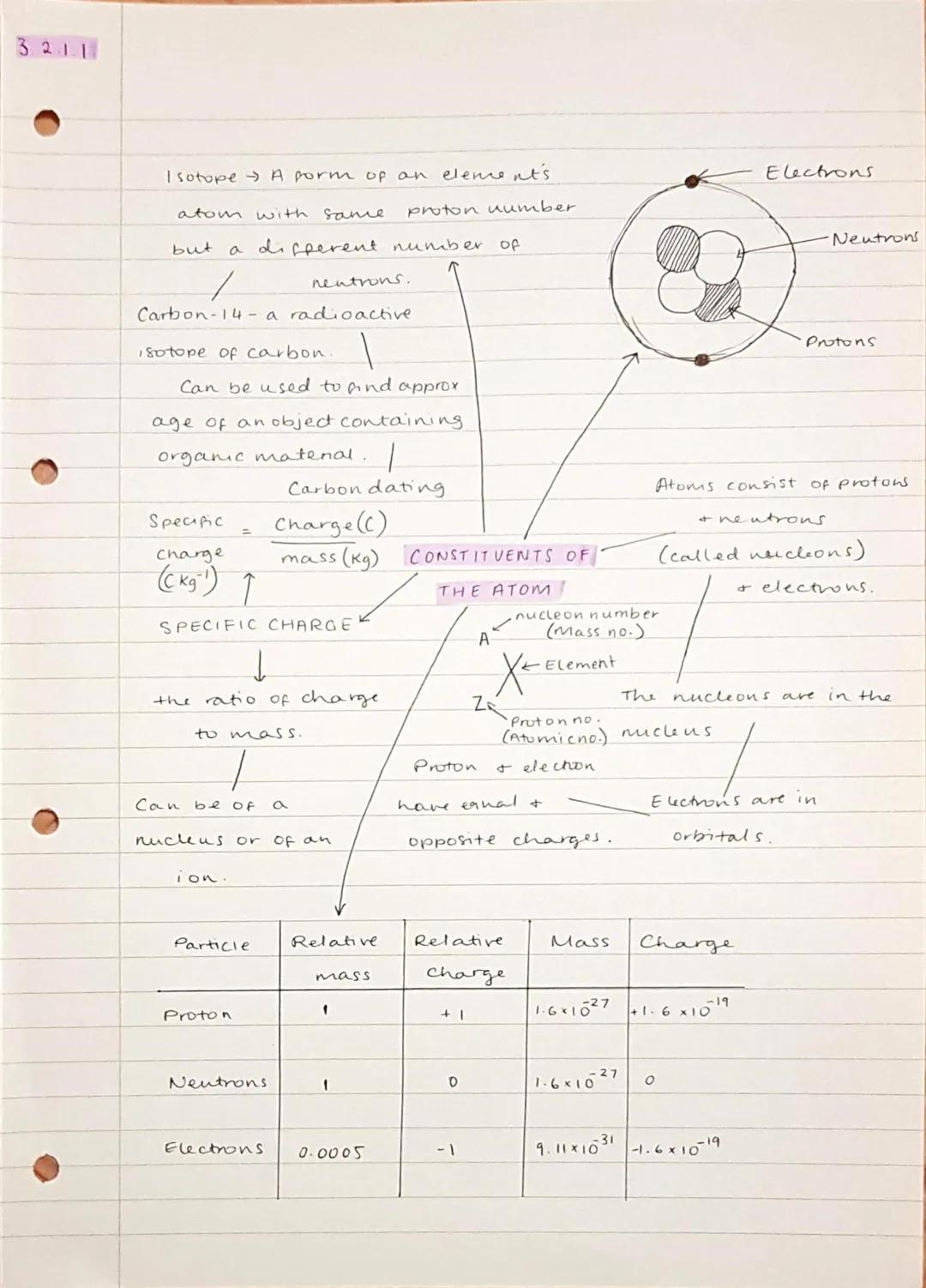
Your atoms are made of three key particles: protons (positive charge), neutrons (no charge), and electrons (negative charge). The protons and neutrons hang out together in the nucleus, whilst electrons orbit around in energy levels.
Isotopes are atoms of the same element with different numbers of neutrons. Carbon-14 is a famous radioactive isotope used for carbon dating - it helps archaeologists figure out how old ancient objects are by measuring how much carbon-14 has decayed over time.
Specific charge is simply the ratio of an object's charge to its mass . You'll need to calculate this for different particles using the formula: specific charge = charge ÷ mass. Remember that protons and electrons have equal but opposite charges, whilst neutrons are electrically neutral.
Key Point: The notation ᴬzX tells you everything - A is the mass number , Z is the atomic number (just protons), and X is the element symbol.

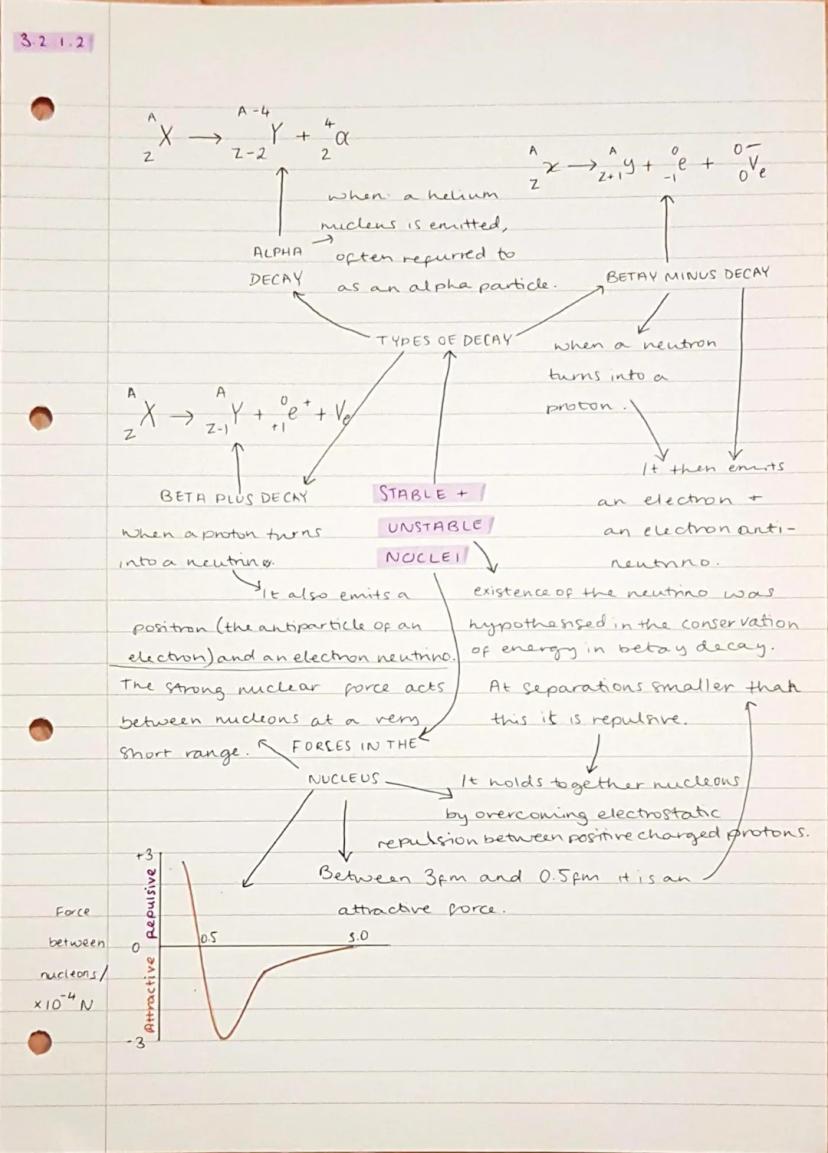
Unstable nuclei undergo radioactive decay to become more stable, and there are three main types you need to know. Alpha decay shoots out a helium nucleus , beta-minus decay turns a neutron into a proton whilst emitting an electron, and beta-plus decay converts a proton into a neutron whilst releasing a positron.
The strong nuclear force is what keeps the nucleus together despite all those positively charged protons trying to repel each other. It's incredibly powerful but only works over tiny distances - between about 0.5 and 3 femtometres. Any closer than 0.5 fm and it actually becomes repulsive, preventing the nucleus from collapsing.
During beta decay, scientists noticed that energy seemed to disappear, which led to the discovery of neutrinos - nearly massless particles that barely interact with anything. They're essential for conserving energy and momentum in these reactions.
Remember: In alpha decay, both the mass number drops by 4 and the atomic number drops by 2, whilst in beta decay, the mass number stays the same but the atomic number changes by ±1.

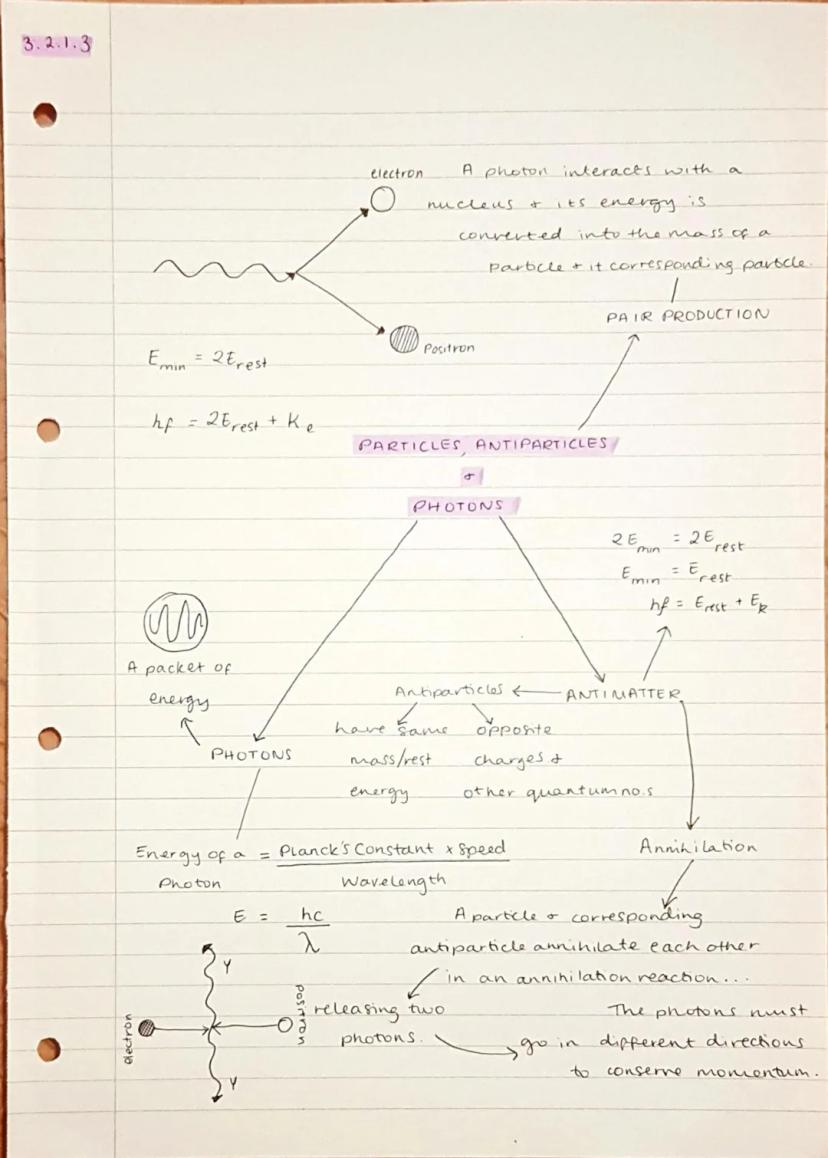
Every particle has a corresponding antiparticle with the same mass but opposite charge - like electrons and positrons. When they meet, they completely destroy each other in an annihilation reaction, converting all their mass into energy as two photons that fly off in opposite directions.
Pair production is the reverse process where a high-energy photon interacts with a nucleus and creates a particle-antiparticle pair. For this to happen, the photon needs at least twice the rest energy of the particles being created .
Photons are packets of electromagnetic energy with energy E = hf (where h is Planck's constant and f is frequency). You can also use E = hc/λ when you know the wavelength instead. These massless particles carry the electromagnetic force and always travel at the speed of light.
Energy Conservation: In pair production, any excess energy above the minimum becomes kinetic energy of the created particles: hf = 2E_rest + KE.

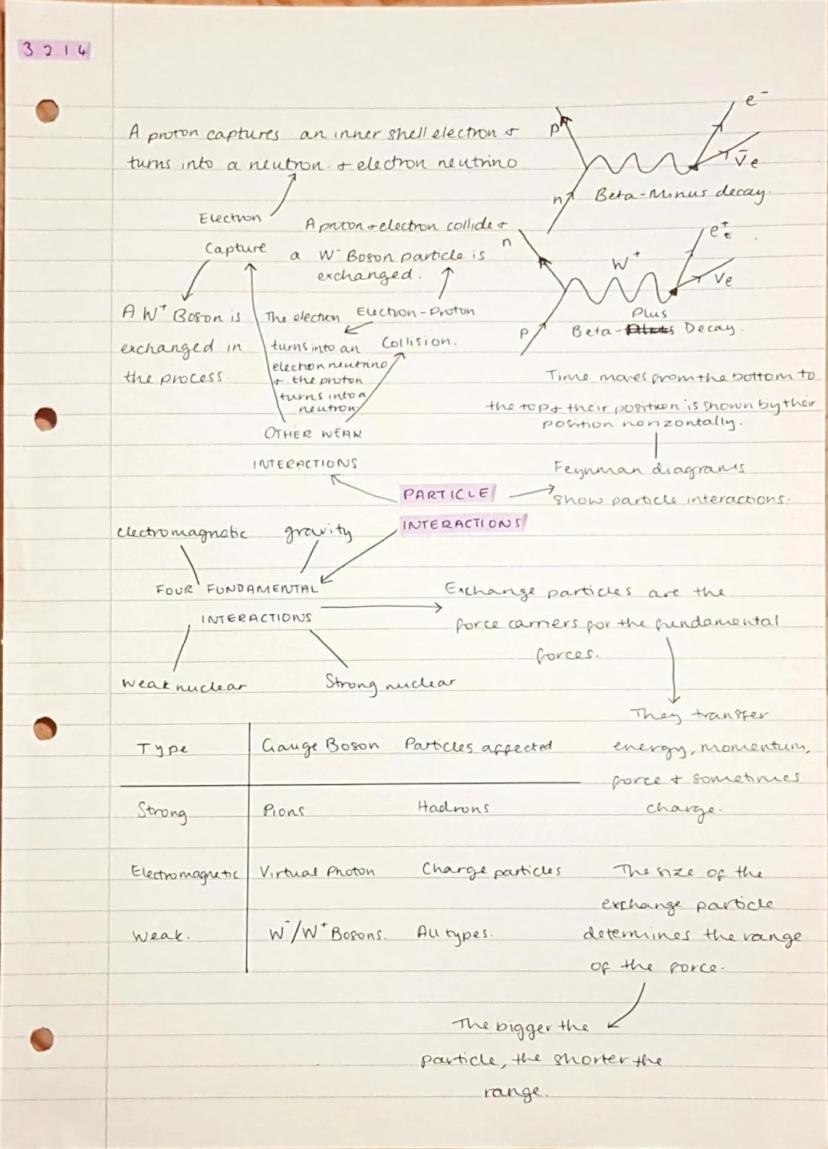
The universe has four fundamental forces: strong nuclear, electromagnetic, weak nuclear, and gravity. Each force is carried by special particles called gauge bosons - think of them as messengers that transfer energy and momentum between particles.
Feynman diagrams are brilliant visual tools that show how particles interact over time (time goes upwards, position goes sideways). In beta decay, a W boson mediates the interaction that converts a neutron to a proton or vice versa. The size of the exchange particle determines the range of the force - bigger particles mean shorter ranges.
Electron capture happens when a proton grabs an inner electron and transforms into a neutron, releasing an electron neutrino. This process involves the weak nuclear force and W bosons, just like other beta decay processes.
The weak nuclear force affects all types of particles and is responsible for radioactive decay processes. Unlike the strong force, it can change one type of particle into another completely.
Force Carriers: Virtual photons carry electromagnetic force, W and Z bosons carry weak force, gluons carry strong force, and gravitons (theoretical) carry gravity.
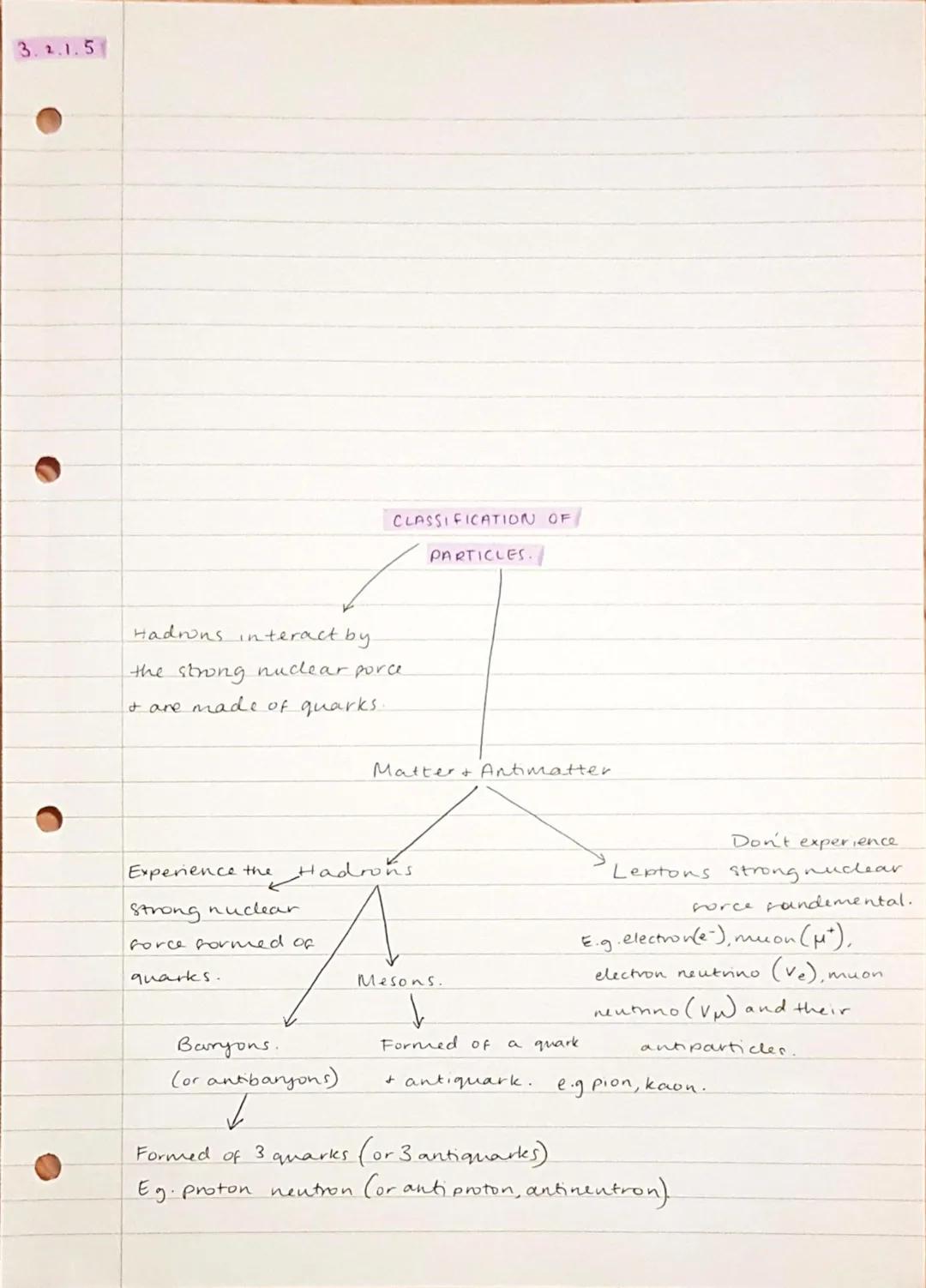
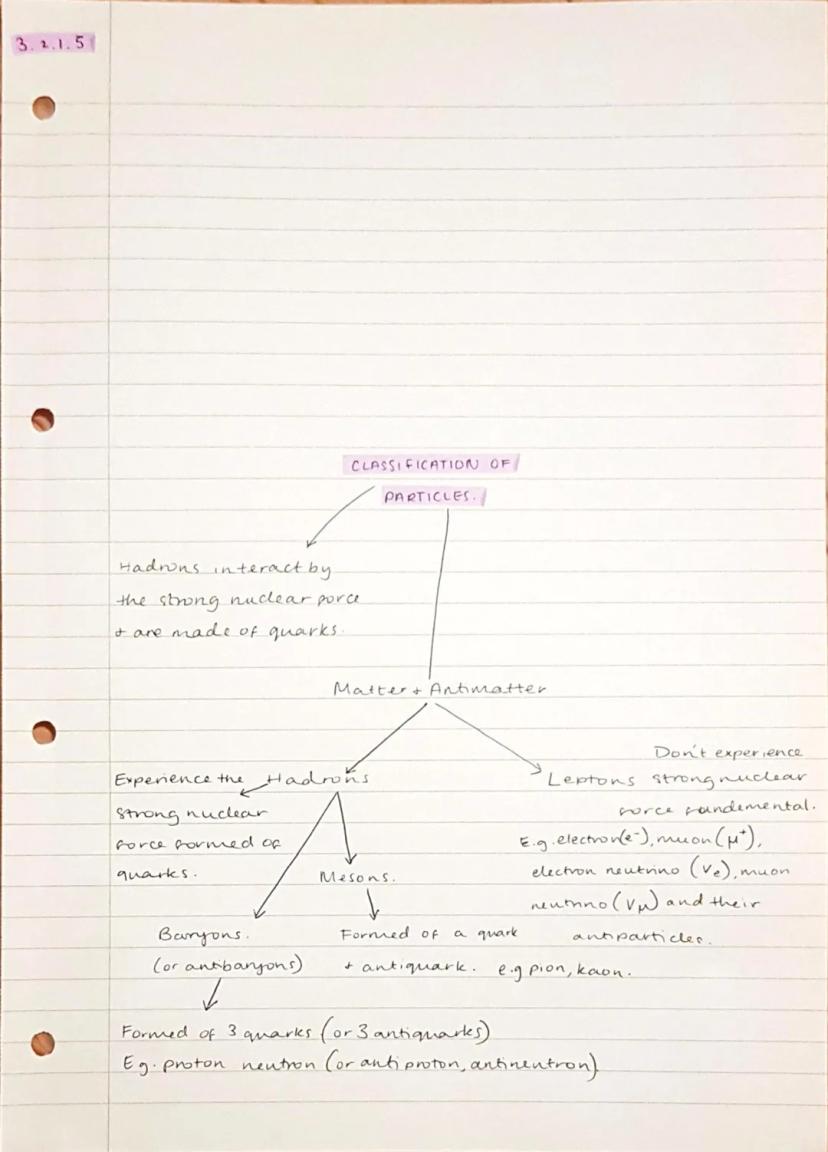
All matter divides into two main categories: hadrons (made of quarks) and leptons (fundamental particles). Hadrons feel the strong nuclear force because they contain quarks, whilst leptons don't experience this force at all.
Hadrons split into two groups: baryons (made of three quarks, like protons and neutrons) and mesons . These particles can interact through the strong force and are generally more massive than leptons.
Leptons include familiar particles like electrons and neutrinos, plus their more exotic cousins like muons. These are truly fundamental - they can't be broken down into smaller components and only interact through electromagnetic, weak, and gravitational forces.
Each particle type has its own antiparticle with identical mass but opposite charge and other quantum numbers. When matter meets antimatter, they annihilate completely, releasing pure energy.
Memory Tip: Think "LEPtons are LIGHTweight and LEft alone by the strong force" - they don't experience strong nuclear interactions.
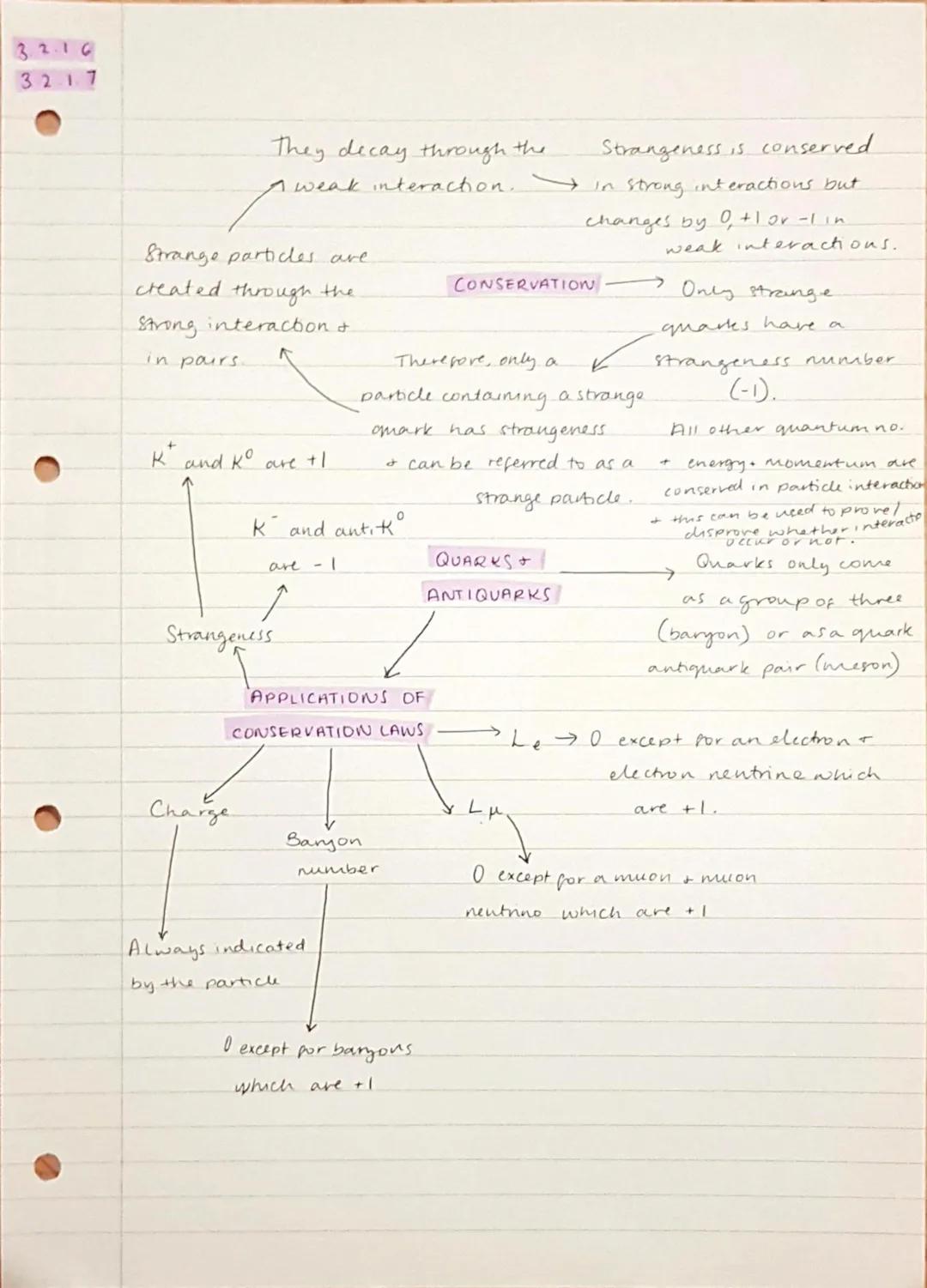
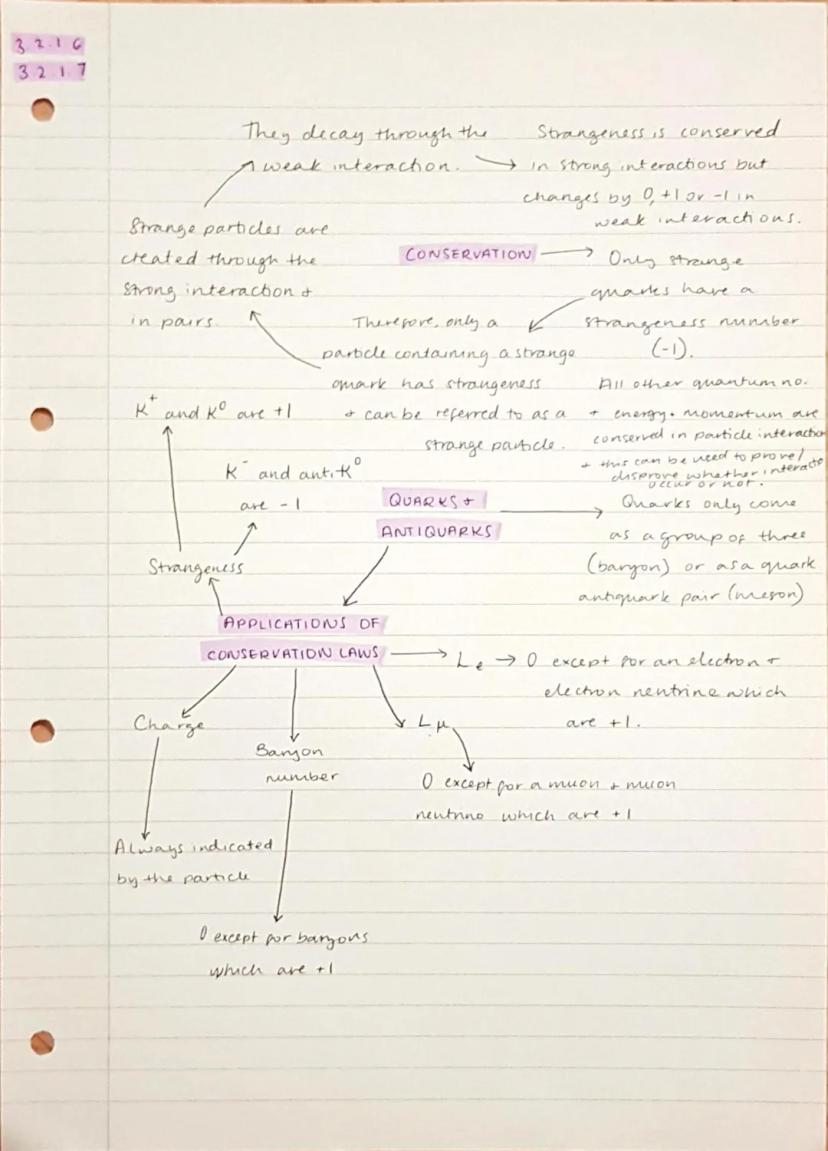
Conservation laws are the universe's accounting rules - certain quantities must balance before and after any interaction. Energy, momentum, charge, and baryon number are always conserved, whilst strangeness is only conserved in strong interactions.
Strange particles contain strange quarks and have non-zero strangeness values. They're created in pairs through strong interactions (to conserve strangeness) but decay individually through weak interactions .
Quarks are the building blocks of hadrons and come in six flavours, but you mainly need to know up, down, and strange quarks for A-level. They combine in groups of three (baryons) or quark-antiquark pairs (mesons) - you never find isolated quarks in nature.
Different lepton numbers exist for each lepton family: electron number and muon number . These are conserved in all interactions, helping you predict what particles can be produced in various processes.
Strangeness Rule: Strange particles are always produced in pairs (to conserve strangeness) but can decay alone because weak interactions allow strangeness to change.
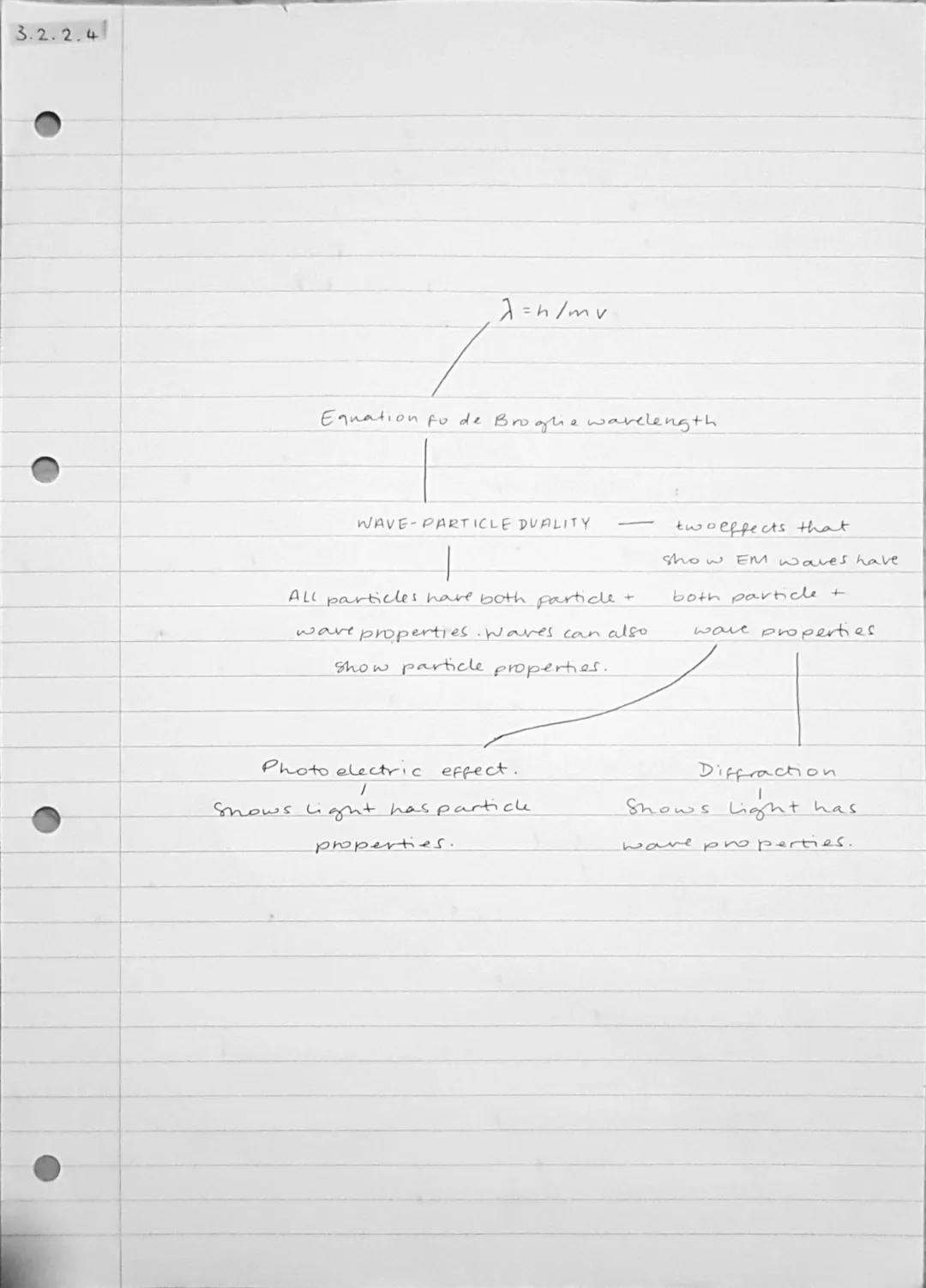
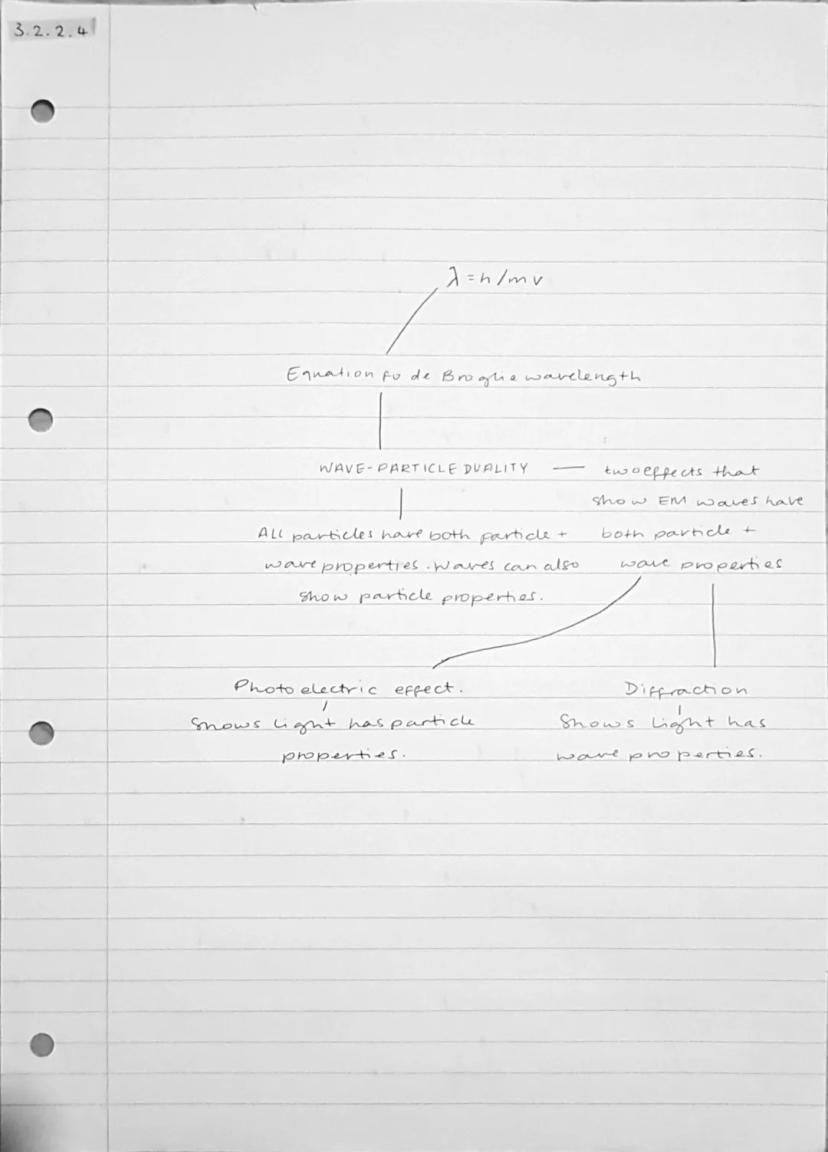
Everything in the universe exhibits wave-particle duality - particles can behave like waves and waves can behave like particles. This isn't just theoretical; it's a fundamental property of matter and energy that you can observe in experiments.
The de Broglie wavelength tells you the wavelength associated with any moving particle. Electrons, protons, even entire atoms have wavelengths, though they're incredibly tiny for large objects. This explains why you don't notice the wave properties of everyday objects.
Light demonstrates this duality perfectly: the photoelectric effect shows light's particle nature (photons), whilst diffraction experiments reveal its wave properties. Both aspects are real and fundamental to how electromagnetic radiation behaves.
Scale Matters: Wave properties become noticeable when the de Broglie wavelength is similar to the size of obstacles or gaps the particle encounters.

When light hits a metal surface, it can knock electrons out - but only if the light's frequency is high enough. This threshold frequency depends on the metal's work function (φ) - the minimum energy needed to free an electron from the metal's surface.
The photoelectric equation E = hf = φ + E_k(max) explains everything. Each photon gives all its energy (hf) to one electron: some energy overcomes the work function, and any leftover becomes the electron's kinetic energy.
Stopping potential is the voltage needed to stop the fastest photoelectrons, related to their maximum kinetic energy by E_k(max) = eV_s. This lets you measure the electrons' energy experimentally and verify Einstein's photon model.
Increasing light intensity (keeping frequency constant) produces more photoelectrons per second but doesn't change their individual energies. Only increasing frequency gives each electron more kinetic energy.
Counter-intuitive: Bright red light won't eject electrons from zinc, but dim ultraviolet light will - frequency matters more than intensity for the photoelectric effect.

Electrons in atoms can only exist at specific discrete energy levels - they can't hang around anywhere in between. When electrons jump between these levels, they absorb or emit photons with energies exactly equal to the energy difference between levels.
Line spectra provide brilliant evidence for these energy levels. Each bright or dark line corresponds to a specific photon frequency, showing that electrons can only make certain allowed transitions. No in-between energies appear because no in-between levels exist.
Ionisation energy is the energy needed to completely remove an electron from an atom's ground state (lowest energy level). Different elements have different ionisation energies because their electron arrangements vary.
The patterns in atomic spectra are unique to each element, making spectroscopy a powerful tool for identifying substances. Astronomers use this to determine what distant stars are made of just by analysing their light.
Think of it like stairs: Electrons can only stand on the steps (energy levels), never floating between them - and they emit or absorb specific amounts of energy when changing steps.

When fast-moving electrons collide with atoms, they can transfer energy to the atom's electrons, causing excitation - bumping electrons up to higher energy levels. The excited electrons quickly fall back down, releasing photons as they cascade through the energy levels.
Electron volts (eV) are handy energy units in atomic physics. One eV equals 1.6 × 10⁻¹⁹ joules - it's the kinetic energy gained by an electron accelerated through a 1V potential difference. Convert between eV and joules by multiplying or dividing by 1.6 × 10⁻¹⁹.
If the colliding electron has enough energy (greater than the ionisation energy), it can completely knock an electron out of the atom, creating an ion. This process requires more energy than just exciting electrons to higher levels.
Fluorescent tubes demonstrate these principles perfectly: high voltage accelerates electrons that collide with mercury atoms, exciting them to emit UV light. The phosphor coating then absorbs the UV and re-emits visible light through its own excitation process.
Energy Threshold: Excitation and ionisation only happen when the colliding electron has at least the exact energy needed - there's no partial credit in quantum mechanics!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
thressi kutty
@thressikutty_naiu
Atomic physics covers the fundamental building blocks of matter and how they behave. You'll explore everything from the structure of atoms and radioactive decay to the dual nature of light and matter, plus some fascinating quantum effects that explain how... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Your atoms are made of three key particles: protons (positive charge), neutrons (no charge), and electrons (negative charge). The protons and neutrons hang out together in the nucleus, whilst electrons orbit around in energy levels.
Isotopes are atoms of the same element with different numbers of neutrons. Carbon-14 is a famous radioactive isotope used for carbon dating - it helps archaeologists figure out how old ancient objects are by measuring how much carbon-14 has decayed over time.
Specific charge is simply the ratio of an object's charge to its mass . You'll need to calculate this for different particles using the formula: specific charge = charge ÷ mass. Remember that protons and electrons have equal but opposite charges, whilst neutrons are electrically neutral.
Key Point: The notation ᴬzX tells you everything - A is the mass number , Z is the atomic number (just protons), and X is the element symbol.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Unstable nuclei undergo radioactive decay to become more stable, and there are three main types you need to know. Alpha decay shoots out a helium nucleus , beta-minus decay turns a neutron into a proton whilst emitting an electron, and beta-plus decay converts a proton into a neutron whilst releasing a positron.
The strong nuclear force is what keeps the nucleus together despite all those positively charged protons trying to repel each other. It's incredibly powerful but only works over tiny distances - between about 0.5 and 3 femtometres. Any closer than 0.5 fm and it actually becomes repulsive, preventing the nucleus from collapsing.
During beta decay, scientists noticed that energy seemed to disappear, which led to the discovery of neutrinos - nearly massless particles that barely interact with anything. They're essential for conserving energy and momentum in these reactions.
Remember: In alpha decay, both the mass number drops by 4 and the atomic number drops by 2, whilst in beta decay, the mass number stays the same but the atomic number changes by ±1.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Every particle has a corresponding antiparticle with the same mass but opposite charge - like electrons and positrons. When they meet, they completely destroy each other in an annihilation reaction, converting all their mass into energy as two photons that fly off in opposite directions.
Pair production is the reverse process where a high-energy photon interacts with a nucleus and creates a particle-antiparticle pair. For this to happen, the photon needs at least twice the rest energy of the particles being created .
Photons are packets of electromagnetic energy with energy E = hf (where h is Planck's constant and f is frequency). You can also use E = hc/λ when you know the wavelength instead. These massless particles carry the electromagnetic force and always travel at the speed of light.
Energy Conservation: In pair production, any excess energy above the minimum becomes kinetic energy of the created particles: hf = 2E_rest + KE.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The universe has four fundamental forces: strong nuclear, electromagnetic, weak nuclear, and gravity. Each force is carried by special particles called gauge bosons - think of them as messengers that transfer energy and momentum between particles.
Feynman diagrams are brilliant visual tools that show how particles interact over time (time goes upwards, position goes sideways). In beta decay, a W boson mediates the interaction that converts a neutron to a proton or vice versa. The size of the exchange particle determines the range of the force - bigger particles mean shorter ranges.
Electron capture happens when a proton grabs an inner electron and transforms into a neutron, releasing an electron neutrino. This process involves the weak nuclear force and W bosons, just like other beta decay processes.
The weak nuclear force affects all types of particles and is responsible for radioactive decay processes. Unlike the strong force, it can change one type of particle into another completely.
Force Carriers: Virtual photons carry electromagnetic force, W and Z bosons carry weak force, gluons carry strong force, and gravitons (theoretical) carry gravity.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
All matter divides into two main categories: hadrons (made of quarks) and leptons (fundamental particles). Hadrons feel the strong nuclear force because they contain quarks, whilst leptons don't experience this force at all.
Hadrons split into two groups: baryons (made of three quarks, like protons and neutrons) and mesons . These particles can interact through the strong force and are generally more massive than leptons.
Leptons include familiar particles like electrons and neutrinos, plus their more exotic cousins like muons. These are truly fundamental - they can't be broken down into smaller components and only interact through electromagnetic, weak, and gravitational forces.
Each particle type has its own antiparticle with identical mass but opposite charge and other quantum numbers. When matter meets antimatter, they annihilate completely, releasing pure energy.
Memory Tip: Think "LEPtons are LIGHTweight and LEft alone by the strong force" - they don't experience strong nuclear interactions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Conservation laws are the universe's accounting rules - certain quantities must balance before and after any interaction. Energy, momentum, charge, and baryon number are always conserved, whilst strangeness is only conserved in strong interactions.
Strange particles contain strange quarks and have non-zero strangeness values. They're created in pairs through strong interactions (to conserve strangeness) but decay individually through weak interactions .
Quarks are the building blocks of hadrons and come in six flavours, but you mainly need to know up, down, and strange quarks for A-level. They combine in groups of three (baryons) or quark-antiquark pairs (mesons) - you never find isolated quarks in nature.
Different lepton numbers exist for each lepton family: electron number and muon number . These are conserved in all interactions, helping you predict what particles can be produced in various processes.
Strangeness Rule: Strange particles are always produced in pairs (to conserve strangeness) but can decay alone because weak interactions allow strangeness to change.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Everything in the universe exhibits wave-particle duality - particles can behave like waves and waves can behave like particles. This isn't just theoretical; it's a fundamental property of matter and energy that you can observe in experiments.
The de Broglie wavelength tells you the wavelength associated with any moving particle. Electrons, protons, even entire atoms have wavelengths, though they're incredibly tiny for large objects. This explains why you don't notice the wave properties of everyday objects.
Light demonstrates this duality perfectly: the photoelectric effect shows light's particle nature (photons), whilst diffraction experiments reveal its wave properties. Both aspects are real and fundamental to how electromagnetic radiation behaves.
Scale Matters: Wave properties become noticeable when the de Broglie wavelength is similar to the size of obstacles or gaps the particle encounters.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When light hits a metal surface, it can knock electrons out - but only if the light's frequency is high enough. This threshold frequency depends on the metal's work function (φ) - the minimum energy needed to free an electron from the metal's surface.
The photoelectric equation E = hf = φ + E_k(max) explains everything. Each photon gives all its energy (hf) to one electron: some energy overcomes the work function, and any leftover becomes the electron's kinetic energy.
Stopping potential is the voltage needed to stop the fastest photoelectrons, related to their maximum kinetic energy by E_k(max) = eV_s. This lets you measure the electrons' energy experimentally and verify Einstein's photon model.
Increasing light intensity (keeping frequency constant) produces more photoelectrons per second but doesn't change their individual energies. Only increasing frequency gives each electron more kinetic energy.
Counter-intuitive: Bright red light won't eject electrons from zinc, but dim ultraviolet light will - frequency matters more than intensity for the photoelectric effect.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrons in atoms can only exist at specific discrete energy levels - they can't hang around anywhere in between. When electrons jump between these levels, they absorb or emit photons with energies exactly equal to the energy difference between levels.
Line spectra provide brilliant evidence for these energy levels. Each bright or dark line corresponds to a specific photon frequency, showing that electrons can only make certain allowed transitions. No in-between energies appear because no in-between levels exist.
Ionisation energy is the energy needed to completely remove an electron from an atom's ground state (lowest energy level). Different elements have different ionisation energies because their electron arrangements vary.
The patterns in atomic spectra are unique to each element, making spectroscopy a powerful tool for identifying substances. Astronomers use this to determine what distant stars are made of just by analysing their light.
Think of it like stairs: Electrons can only stand on the steps (energy levels), never floating between them - and they emit or absorb specific amounts of energy when changing steps.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When fast-moving electrons collide with atoms, they can transfer energy to the atom's electrons, causing excitation - bumping electrons up to higher energy levels. The excited electrons quickly fall back down, releasing photons as they cascade through the energy levels.
Electron volts (eV) are handy energy units in atomic physics. One eV equals 1.6 × 10⁻¹⁹ joules - it's the kinetic energy gained by an electron accelerated through a 1V potential difference. Convert between eV and joules by multiplying or dividing by 1.6 × 10⁻¹⁹.
If the colliding electron has enough energy (greater than the ionisation energy), it can completely knock an electron out of the atom, creating an ion. This process requires more energy than just exciting electrons to higher levels.
Fluorescent tubes demonstrate these principles perfectly: high voltage accelerates electrons that collide with mercury atoms, exciting them to emit UV light. The phosphor coating then absorbs the UV and re-emits visible light through its own excitation process.
Energy Threshold: Excitation and ionisation only happen when the colliding electron has at least the exact energy needed - there's no partial credit in quantum mechanics!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
3
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore key concepts in Cosmology for Physics NAT5, including definitions of stars, planets, and galaxies, the Big Bang Theory, light years, and the electromagnetic spectrum. This summary provides essential insights and worked examples to enhance your understanding of the universe's structure and origins.
Comprehensive revision notes covering key concepts in AQA A Level Physics, focusing on Particles and Radiation. Explore topics such as the photoelectric effect, radioactivity, atomic particles, and quantum energy levels. Ideal for Year 12 and Year 13 students preparing for exams.
Explore the essential concepts of particle physics, including the four fundamental forces, types of subatomic particles, and the structure of matter. This summary covers key topics such as quarks, leptons, hadrons, and mass-energy equivalence, providing a clear understanding for A/S Physics students. Ideal for revision and exam preparation.
Explore the fundamental concepts of particle physics, including the structure of atoms, the classification of subatomic particles (hadrons, leptons, baryons, and mesons), and the significance of isotopes. This summary provides essential equations for calculating specific charge and examples of various particles, making it a valuable resource for A-Level physics students.
Explore the core concepts of particle physics, including the four fundamental forces, classification of particles, nuclear decay processes, and the role of quarks. This summary covers essential topics such as protons, neutrons, photons, and conservation laws, providing a comprehensive overview for A-level Physics students.
Dive into the fundamentals of electric fields, including electric potential, forces, and their relationship with gravitational fields. This summary covers key concepts such as Coulomb's law, electric potential energy, and the behavior of charged particles in uniform fields. Ideal for students seeking a clear understanding of electrostatics.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user