This unit covers the essential chemistry of acids, bases, and... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
46
•
11 Feb 2026
•
sunshine
@sunrise_umek
This unit covers the essential chemistry of acids, bases, and... Show more










Ever wondered why antacids work or how farmers fix acidic soil? It all comes down to understanding how acids and bases interact with each other.
Acids are substances that release H+ ions in solution, whilst bases are compounds that accept these H+ ions (protons). When they meet, they neutralise each other in a reaction that always produces salt and water. It's like a chemical handshake that creates something entirely new.
Alkalis are simply bases that dissolve in water to form hydroxide ions . Think of them as water-loving bases that are particularly good at neutralising acids.
Real-world connection: Metal oxides like calcium oxide (lime) are used by farmers to neutralise acidic soil, helping plants grow better by preventing them from absorbing toxic ions.

Your medicine cabinet contains more chemistry than you might think! Metal hydroxides are brilliant at accepting protons and neutralising acids, which makes them perfect for treating stomach problems.
Magnesium hydroxide works as an antacid by neutralising the hydrochloric acid that causes heartburn. Meanwhile, calcium hydroxide is used in industry to clean up acidic waste water - it's cheap and incredibly effective.
The Brønsted-Lowry theory gives us a clearer picture of how acids and bases work. An acid donates a proton whilst a base accepts it. What's clever is that after the reaction, the acid becomes a conjugate base and the base becomes a conjugate acid - they've essentially swapped roles.
Amphoteric substances are the chameleons of chemistry - they can act as either acids or bases depending on what they're reacting with. Water is a perfect example of this flexibility.
Test tip: Remember that amphoteric substances like aluminium oxide can react as both acids and bases - this often comes up in exam questions about reaction conditions.

Creating salts is like following a naming recipe - the first part comes from the metal, and the second part comes from the acid. Hydrochloric acid creates chlorides, nitric acid makes nitrates, and sulfuric acid produces sulphates.
Alumina (aluminium oxide, Al₂O₃) is crucial for producing aluminium metal. It starts life as bauxite, which contains hydrated aluminium oxides mixed with impurities like iron oxide and silica that must be removed.
The challenge is getting pure alumina because any impurities would contaminate the final aluminium product. This purity requirement drives the entire extraction process.
Industry insight: Alumina's high-temperature stability makes it perfect for lining furnaces and kilns - materials that need to stay strong under extreme heat are called refractories.

The Bayer Process is an elegant solution to extracting pure alumina from messy bauxite ore. It's like a sophisticated filtering and purification system that uses chemistry instead of just physical separation.
The process starts by crushing bauxite and treating it with caustic soda (NaOH) at 170°C. This creates soluble sodium aluminate whilst leaving the impurities behind as an insoluble residue that can be filtered out.
The clean solution is then cooled and crystallised to form aluminium hydroxide, which is finally heated in a rotary kiln to produce pure aluminium oxide. Most of this alumina heads straight to the Hall-Héroult process for aluminium production.
The leftover red mud is an environmental concern, but the process efficiently separates valuable aluminium from worthless impurities on an industrial scale.
Process tip: The key temperatures (170°C for reaction, high heat for final conversion) and the role of NaOH are frequently tested - make sure you understand why each step matters.

The electrochemical series is like a league table for elements, ranking them by how easily they give up electrons. More reactive elements at the top form ions readily, whilst less reactive ones at the bottom prefer to stay as atoms.
Electrolysis only works when ions can move freely, which happens in molten ionic compounds or solutions. Solid ionic compounds are useless because their ions are locked in fixed positions.
During electrolysis, cations (positive ions) head to the cathode (negative electrode) where they gain electrons, whilst anions (negative ions) move to the anode (positive electrode) where they lose electrons. It's like a perfectly organised electrical dance.
Understanding this movement is crucial because it determines what products you'll get from any electrolysis reaction.
Memory trick: Remember "PANIC" - Positive Anode, Negative Is Cathode. This helps you keep the electrodes straight during electrolysis problems.

Electrolysis gets more complex with aqueous solutions because water molecules also dissociate into H+ and OH- ions, giving you more options at each electrode.
The products depend entirely on the reactivity of the elements involved. At the cathode, if the metal ions are more reactive than hydrogen, they stay in solution and hydrogen gas forms instead. It's like the less reactive element wins the race to the electrode.
At the anode, halide ions get oxidised to produce halogen atoms if they're present. Otherwise, OH- ions get discharged to produce oxygen and water. The electrochemical series tells you which reaction will actually happen.
This selectivity is what makes electrolysis so useful for industrial processes - you can predict and control what products you'll get.
Exam focus: Questions often ask you to predict electrolysis products - always check the reactivity series and remember that less reactive elements get discharged first.

The membrane cell revolutionised chlorine production by solving the problem of keeping products separate. Its ion-exchange membrane acts like a selective door that only allows sodium ions through whilst blocking chloride ions.
This selectivity is crucial because it prevents contamination of the sodium hydroxide product with sodium chloride. The brine enters from the anode side, and the pure caustic solution exits from the cathode side.
At the cathode, hydrogen gas bubbles out as water splits, whilst chlorine gas forms at the anode. The membrane ensures these potentially explosive gases never meet inside the cell.
The sodium hydroxide leaves at about 30% concentration and is often concentrated further to 50% for commercial use. The chlorine gas requires purification through liquefaction and evaporation to remove oxygen impurities.
Safety note: Keeping chlorine and hydrogen separate is critical - their mixture explodes violently when exposed to sunlight or heat, producing dangerous hydrogen chloride gas.

The membrane cell represents the cutting edge of chlorine production technology, though it comes with trade-offs that affect industrial decisions.
Advantages: The high purity of sodium hydroxide produced makes it valuable for many applications. The membrane needs minimal maintenance once installed, and the cell uses less energy per tonne of chlorine - making it more environmentally friendly.
Disadvantages: The higher construction costs can be prohibitive for some operations. However, the long-term benefits often outweigh these initial expenses.
The polymer membrane is a significant improvement over older materials, lasting much longer and performing more reliably than previous technologies.
Economic insight: While membrane cells cost more upfront, their lower energy consumption and higher product purity often make them more profitable in the long run.

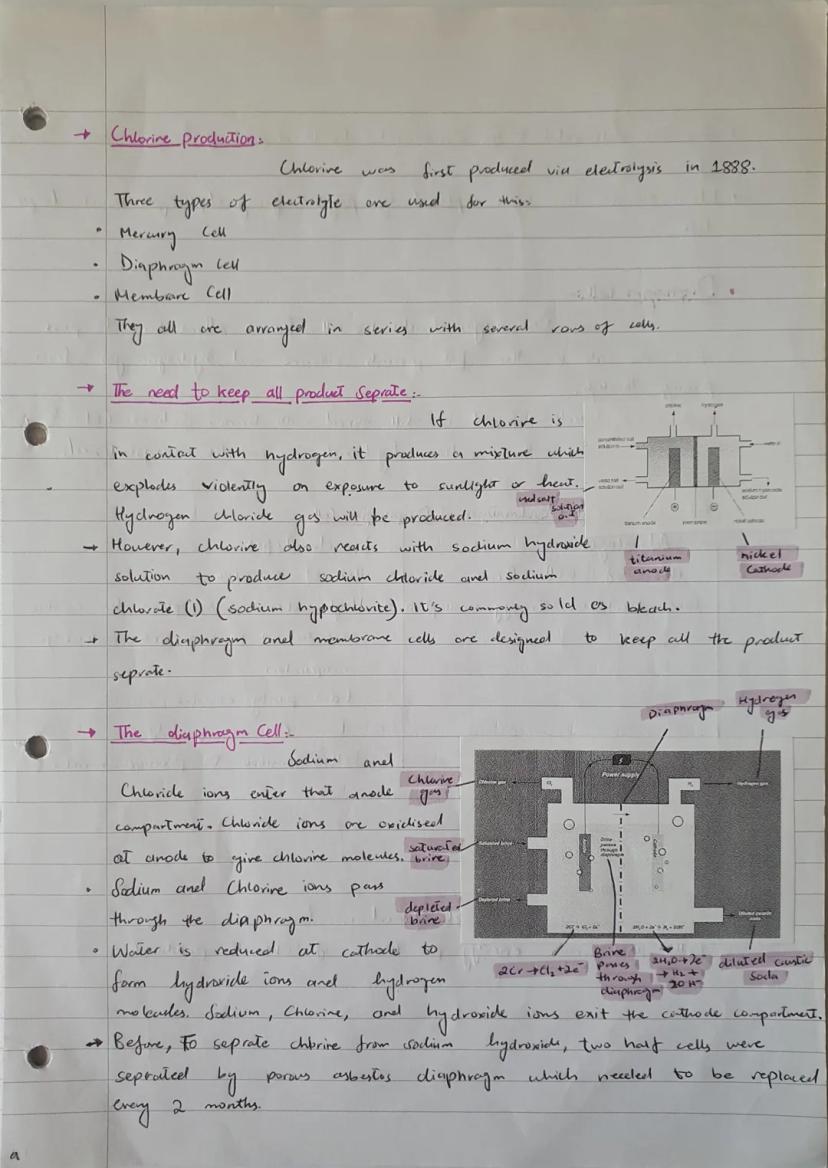
Chlorine production has evolved significantly since its first electrolytic production in 1888. Three main cell types dominate the industry: mercury cells, diaphragm cells, and membrane cells, all arranged in series for maximum efficiency.
The critical challenge is keeping all products separate. When chlorine contacts hydrogen, it creates an explosively dangerous mixture. When chlorine meets sodium hydroxide solution, it produces sodium hypochlorite - the active ingredient in household bleach.
Diaphragm cells use a porous barrier that allows brine to pass through whilst preventing gas mixing. Sodium and chloride ions enter the anode compartment, where chloride gets oxidised to chlorine gas. Meanwhile, water reduction at the cathode produces hydrogen and hydroxide ions.
The old asbestos diaphragms needed replacing every two months and caused environmental problems. Modern polymer diaphragms last much longer and are environmentally safer.
Historical note: The replacement of asbestos with polymers shows how industrial chemistry evolves to become safer and more sustainable over time.

Diaphragm cells offer a different approach to chlorine production with their own advantages and challenges. The porous diaphragm allows brine flow whilst preventing dangerous gas mixing, but the sodium hydroxide product remains mixed with brine.
This contamination means extra processing - sodium chloride is less soluble than sodium hydroxide, so it can be removed by recrystallisation. However, this extra step reduces the purity compared to membrane cells.
Advantages include: Lower construction costs and the ability to use impure brine make diaphragm cells attractive for some operations.
Disadvantages are significant: Higher energy consumption per tonne of chlorine, lower product purity, and sensitivity to pressure variations make them less efficient than membrane cells. The need for regular diaphragm replacement adds maintenance costs.
The choice between diaphragm and membrane cells often comes down to balancing initial costs against long-term operating efficiency and product quality requirements.
Decision factor: Companies must weigh cheaper construction costs against higher operating expenses and lower product quality when choosing cell technology.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
sunshine
@sunrise_umek
This unit covers the essential chemistry of acids, bases, and how they interact to create useful substances in everyday life. You'll discover how these reactions power everything from treating heartburn to producing chlorine gas for water treatment, making this knowledge... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why antacids work or how farmers fix acidic soil? It all comes down to understanding how acids and bases interact with each other.
Acids are substances that release H+ ions in solution, whilst bases are compounds that accept these H+ ions (protons). When they meet, they neutralise each other in a reaction that always produces salt and water. It's like a chemical handshake that creates something entirely new.
Alkalis are simply bases that dissolve in water to form hydroxide ions . Think of them as water-loving bases that are particularly good at neutralising acids.
Real-world connection: Metal oxides like calcium oxide (lime) are used by farmers to neutralise acidic soil, helping plants grow better by preventing them from absorbing toxic ions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Your medicine cabinet contains more chemistry than you might think! Metal hydroxides are brilliant at accepting protons and neutralising acids, which makes them perfect for treating stomach problems.
Magnesium hydroxide works as an antacid by neutralising the hydrochloric acid that causes heartburn. Meanwhile, calcium hydroxide is used in industry to clean up acidic waste water - it's cheap and incredibly effective.
The Brønsted-Lowry theory gives us a clearer picture of how acids and bases work. An acid donates a proton whilst a base accepts it. What's clever is that after the reaction, the acid becomes a conjugate base and the base becomes a conjugate acid - they've essentially swapped roles.
Amphoteric substances are the chameleons of chemistry - they can act as either acids or bases depending on what they're reacting with. Water is a perfect example of this flexibility.
Test tip: Remember that amphoteric substances like aluminium oxide can react as both acids and bases - this often comes up in exam questions about reaction conditions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Creating salts is like following a naming recipe - the first part comes from the metal, and the second part comes from the acid. Hydrochloric acid creates chlorides, nitric acid makes nitrates, and sulfuric acid produces sulphates.
Alumina (aluminium oxide, Al₂O₃) is crucial for producing aluminium metal. It starts life as bauxite, which contains hydrated aluminium oxides mixed with impurities like iron oxide and silica that must be removed.
The challenge is getting pure alumina because any impurities would contaminate the final aluminium product. This purity requirement drives the entire extraction process.
Industry insight: Alumina's high-temperature stability makes it perfect for lining furnaces and kilns - materials that need to stay strong under extreme heat are called refractories.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The Bayer Process is an elegant solution to extracting pure alumina from messy bauxite ore. It's like a sophisticated filtering and purification system that uses chemistry instead of just physical separation.
The process starts by crushing bauxite and treating it with caustic soda (NaOH) at 170°C. This creates soluble sodium aluminate whilst leaving the impurities behind as an insoluble residue that can be filtered out.
The clean solution is then cooled and crystallised to form aluminium hydroxide, which is finally heated in a rotary kiln to produce pure aluminium oxide. Most of this alumina heads straight to the Hall-Héroult process for aluminium production.
The leftover red mud is an environmental concern, but the process efficiently separates valuable aluminium from worthless impurities on an industrial scale.
Process tip: The key temperatures (170°C for reaction, high heat for final conversion) and the role of NaOH are frequently tested - make sure you understand why each step matters.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The electrochemical series is like a league table for elements, ranking them by how easily they give up electrons. More reactive elements at the top form ions readily, whilst less reactive ones at the bottom prefer to stay as atoms.
Electrolysis only works when ions can move freely, which happens in molten ionic compounds or solutions. Solid ionic compounds are useless because their ions are locked in fixed positions.
During electrolysis, cations (positive ions) head to the cathode (negative electrode) where they gain electrons, whilst anions (negative ions) move to the anode (positive electrode) where they lose electrons. It's like a perfectly organised electrical dance.
Understanding this movement is crucial because it determines what products you'll get from any electrolysis reaction.
Memory trick: Remember "PANIC" - Positive Anode, Negative Is Cathode. This helps you keep the electrodes straight during electrolysis problems.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrolysis gets more complex with aqueous solutions because water molecules also dissociate into H+ and OH- ions, giving you more options at each electrode.
The products depend entirely on the reactivity of the elements involved. At the cathode, if the metal ions are more reactive than hydrogen, they stay in solution and hydrogen gas forms instead. It's like the less reactive element wins the race to the electrode.
At the anode, halide ions get oxidised to produce halogen atoms if they're present. Otherwise, OH- ions get discharged to produce oxygen and water. The electrochemical series tells you which reaction will actually happen.
This selectivity is what makes electrolysis so useful for industrial processes - you can predict and control what products you'll get.
Exam focus: Questions often ask you to predict electrolysis products - always check the reactivity series and remember that less reactive elements get discharged first.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The membrane cell revolutionised chlorine production by solving the problem of keeping products separate. Its ion-exchange membrane acts like a selective door that only allows sodium ions through whilst blocking chloride ions.
This selectivity is crucial because it prevents contamination of the sodium hydroxide product with sodium chloride. The brine enters from the anode side, and the pure caustic solution exits from the cathode side.
At the cathode, hydrogen gas bubbles out as water splits, whilst chlorine gas forms at the anode. The membrane ensures these potentially explosive gases never meet inside the cell.
The sodium hydroxide leaves at about 30% concentration and is often concentrated further to 50% for commercial use. The chlorine gas requires purification through liquefaction and evaporation to remove oxygen impurities.
Safety note: Keeping chlorine and hydrogen separate is critical - their mixture explodes violently when exposed to sunlight or heat, producing dangerous hydrogen chloride gas.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The membrane cell represents the cutting edge of chlorine production technology, though it comes with trade-offs that affect industrial decisions.
Advantages: The high purity of sodium hydroxide produced makes it valuable for many applications. The membrane needs minimal maintenance once installed, and the cell uses less energy per tonne of chlorine - making it more environmentally friendly.
Disadvantages: The higher construction costs can be prohibitive for some operations. However, the long-term benefits often outweigh these initial expenses.
The polymer membrane is a significant improvement over older materials, lasting much longer and performing more reliably than previous technologies.
Economic insight: While membrane cells cost more upfront, their lower energy consumption and higher product purity often make them more profitable in the long run.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chlorine production has evolved significantly since its first electrolytic production in 1888. Three main cell types dominate the industry: mercury cells, diaphragm cells, and membrane cells, all arranged in series for maximum efficiency.
The critical challenge is keeping all products separate. When chlorine contacts hydrogen, it creates an explosively dangerous mixture. When chlorine meets sodium hydroxide solution, it produces sodium hypochlorite - the active ingredient in household bleach.
Diaphragm cells use a porous barrier that allows brine to pass through whilst preventing gas mixing. Sodium and chloride ions enter the anode compartment, where chloride gets oxidised to chlorine gas. Meanwhile, water reduction at the cathode produces hydrogen and hydroxide ions.
The old asbestos diaphragms needed replacing every two months and caused environmental problems. Modern polymer diaphragms last much longer and are environmentally safer.
Historical note: The replacement of asbestos with polymers shows how industrial chemistry evolves to become safer and more sustainable over time.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Diaphragm cells offer a different approach to chlorine production with their own advantages and challenges. The porous diaphragm allows brine flow whilst preventing dangerous gas mixing, but the sodium hydroxide product remains mixed with brine.
This contamination means extra processing - sodium chloride is less soluble than sodium hydroxide, so it can be removed by recrystallisation. However, this extra step reduces the purity compared to membrane cells.
Advantages include: Lower construction costs and the ability to use impure brine make diaphragm cells attractive for some operations.
Disadvantages are significant: Higher energy consumption per tonne of chlorine, lower product purity, and sensitivity to pressure variations make them less efficient than membrane cells. The need for regular diaphragm replacement adds maintenance costs.
The choice between diaphragm and membrane cells often comes down to balancing initial costs against long-term operating efficiency and product quality requirements.
Decision factor: Companies must weigh cheaper construction costs against higher operating expenses and lower product quality when choosing cell technology.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
2
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user