Time to dive into organic chemistry and discover how carbon... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
390
•
3 Feb 2026
•
Amy Neill
@amyneill
Time to dive into organic chemistry and discover how carbon... Show more










This is your gateway to understanding organic chemistry - the study of carbon-based compounds that make up everything from petrol to perfumes. You'll master the art of naming molecules and recognising patterns in their behaviour.
The key to success here is learning to spot the structural patterns that determine how molecules behave. Once you've cracked the code, you'll see chemistry everywhere around you.
Quick Tip: Keep the naming rules handy - they're your roadmap through this entire topic!

Ever wondered why petrol burns differently to cooking oil? It's all about homologous series - families of compounds that share similar structures and properties. Think of them as chemical cousins with the same general formula but different sizes.
The main families you need to know are alkanes (like methane), alkenes (like ethene), cycloalkanes, alcohols, and carboxylic acids. Each has its own general formula that acts like a mathematical recipe for building molecules.
When naming branched molecules, follow the four golden rules: find the longest chain, identify branches, number carbons to get the lowest numbers, then list branches alphabetically. Remember "McBride Educates People By Pretending He Has Oscars" for the carbon chain lengths.
Isomers are like molecular twins - same formula, different arrangement. They're crucial because even tiny structural changes can completely alter a compound's properties.
Memory Hook: Use the mnemonic to nail those carbon chain prefixes - it'll save you marks in exams!

Here's where chemistry gets exciting - saturated compounds are like fully packed suitcases (only single bonds), while unsaturated compounds have room for more (contain double bonds). This difference completely changes how they react.
The bromine test is your go-to method for spotting unsaturated compounds. Add bromine water and watch - if it goes from orange to colourless, you've got double bonds present. It's like a chemical detective test.
Addition reactions happen when small molecules crash into double bonds and stick there. Whether it's bromination, hydrogenation, chlorination, or hydration, the pattern is always the same - the double bond breaks and new atoms join the party.
Understanding these reactions isn't just academic - they're used to make everything from margarine (hydrogenation) to plastics (polymerisation).
Real World: Hydrogenation turns liquid oils into solid margarine - that's addition reactions in your kitchen!
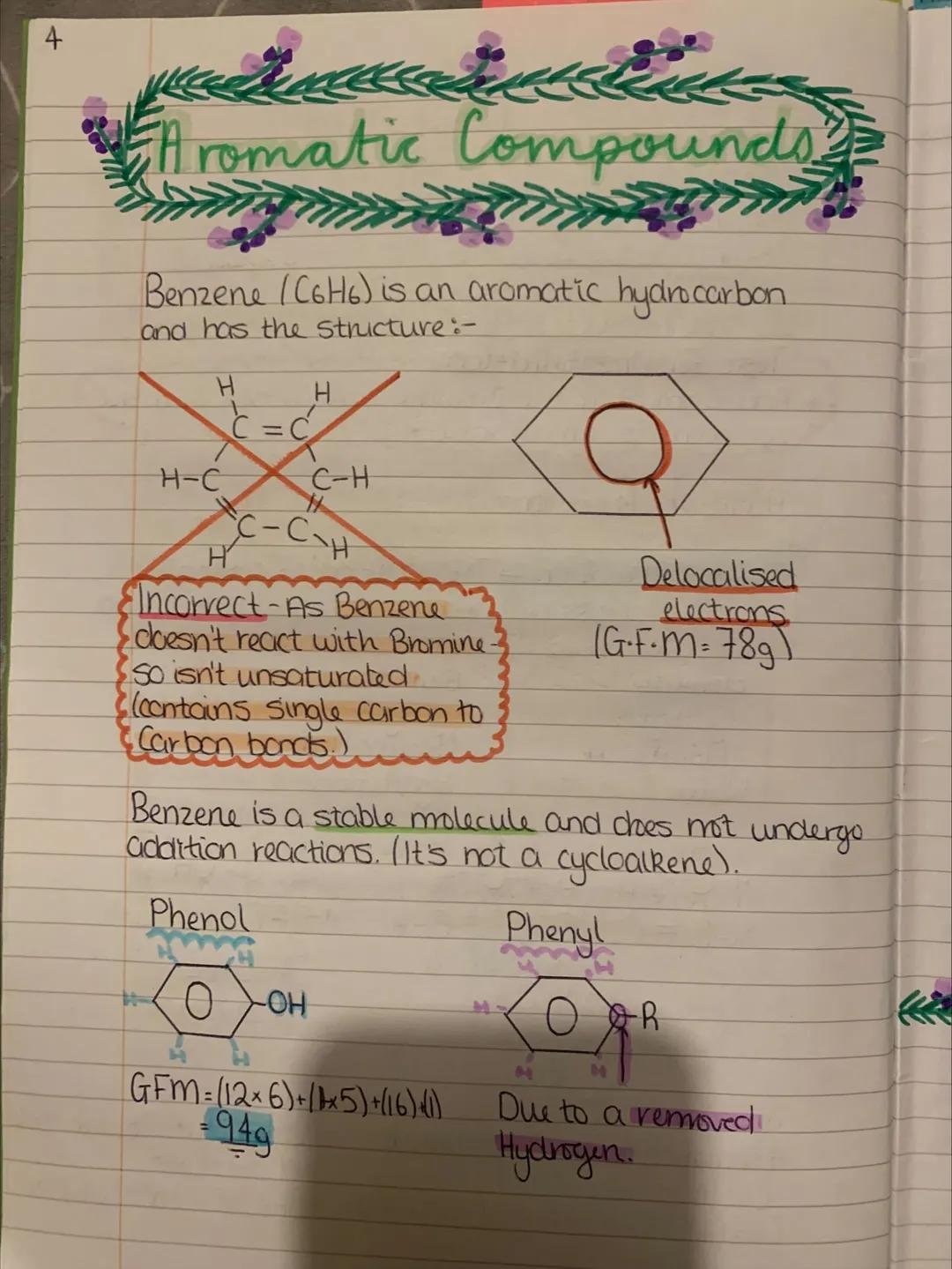
Meet benzene - chemistry's rebel that breaks all the rules you've just learned. Despite looking like it has double bonds, benzene doesn't behave like other unsaturated compounds. It's got delocalised electrons that make it incredibly stable.
This stability means benzene won't react with bromine like other unsaturated compounds. Think of it as chemistry's equivalent of a perfectly balanced spinning top - it's happy as it is and doesn't want to change.
Phenol and phenyl groups are benzene's derivatives that pop up everywhere in organic chemistry. Phenol is benzene with an -OH group attached, making it useful for everything from antiseptics to plastics.
The key insight here is that aromatic compounds march to their own beat - they prefer substitution reactions over addition reactions because they want to keep their stable ring structure.
Key Point: Benzene's stability comes from electron delocalisation - imagine electrons spread like butter across the entire ring rather than stuck between specific atoms.

Alcohols are the party molecules of organic chemistry - they contain the hydroxyl group that makes them incredibly versatile. From the ethanol in hand sanitiser to the glycerol in soap, alcohols are everywhere.
The general formula CₙH₂ₙ₊₁OH is your key to identifying any alcohol. But chemistry loves variety, so you'll also meet diols and triols like glycerol.
Glycerol is particularly important - it's the backbone of fats and oils in your body. Understanding its structure helps explain why fats behave the way they do.
The position of that -OH group matters enormously. Moving it just one carbon along the chain can change a molecule from a useful fuel additive to a toxic poison.
Fun Fact: Glycerol is what makes soap slippery and helps keep your skin moisturised!

Not all alcohols are created equal - they come in three distinct types based on where that hydroxyl group attaches. Primary alcohols have the -OH attached to a carbon with only one other carbon neighbour. Think of them as the end-of-the-line molecules.
Secondary alcohols are the middle children - their -OH sits on a carbon with two carbon neighbours. These often make great solvents and appear in many household products.
Tertiary alcohols are the crowded ones - three carbon atoms surround the carbon bearing the -OH group. This crowding makes them behave very differently in reactions, often making them more resistant to oxidation.
Why does this matter? Each type reacts differently when you try to oxidise them, producing different products. Primary alcohols can become carboxylic acids, secondary become ketones, but tertiary alcohols stubbornly refuse to oxidise under normal conditions.
Memory Trick: Primary = one neighbour, Secondary = two neighbours, Tertiary = three neighbours. Simple!

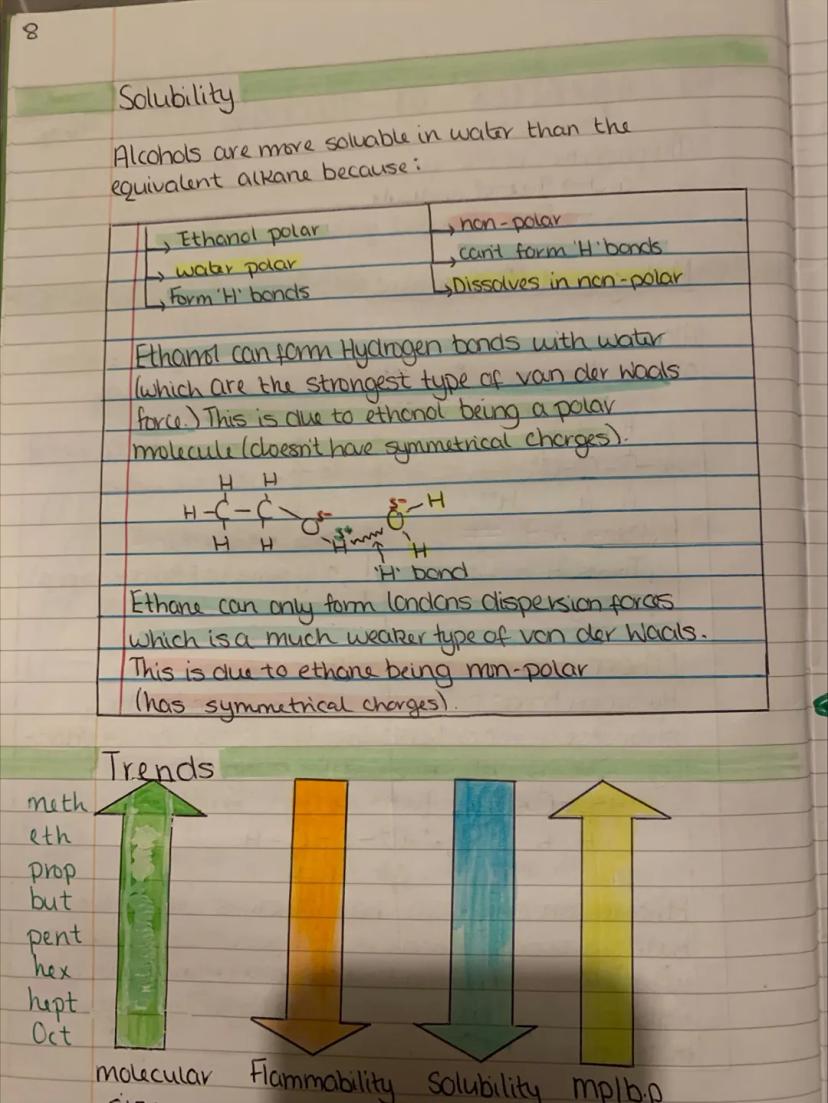
Ever wondered why alcohols have such different properties from their alkane cousins? It all comes down to that polar hydroxyl group and its ability to form hydrogen bonds. These are like molecular handshakes that hold molecules together more tightly.
Melting and boiling points of alcohols are much higher than equivalent alkanes because hydrogen bonds are stronger than the weak London dispersion forces in alkanes. Breaking hydrogen bonds requires more energy, hence higher temperatures.
This explains why ethanol (alcohol) boils at 78°C while ethane boils at -89°C, despite having similar molecular weights. The hydrogen bonding makes all the difference.
As alcohol molecules get larger, they become less polar overall. The hydrocarbon chain grows while the -OH group stays the same size, gradually changing the molecule's personality from polar to non-polar.
Key Insight: Hydrogen bonds are the strongest intermolecular forces - they're what make alcohols so different from hydrocarbons.
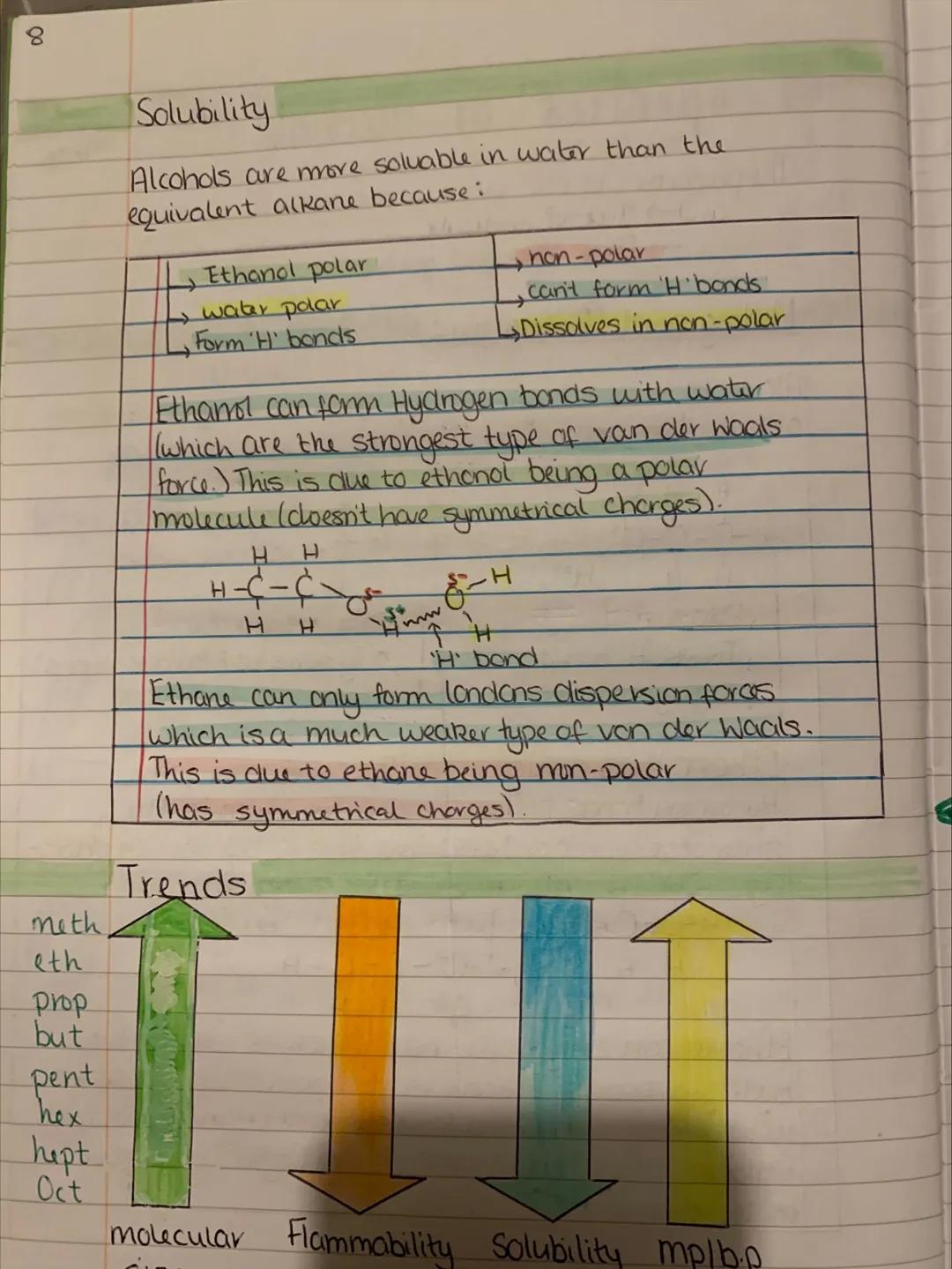
Solubility in water follows a clear pattern - short-chain alcohols dissolve brilliantly because they can form hydrogen bonds with water molecules. But as the carbon chain grows longer, the non-polar hydrocarbon part takes over and solubility drops dramatically.
Ethanol mixes with water in any proportion (that's why alcoholic drinks exist!), but try dissolving octanol and you'll be waiting forever. The long hydrocarbon chain makes it behave more like oil than alcohol.
This creates fascinating trends as you move up the alcohol series. Flammability decreases, molecular mass increases, solubility in water drops, and melting/boiling points rise steadily.
Understanding these trends helps explain why methanol is used as a fuel, ethanol for drinks and antiseptics, and longer alcohols for industrial solvents and lubricants.
Real World: This is why vodka (40% ethanol) mixes with water, but cooking oil doesn't - it's all about polarity and hydrogen bonding!

Carboxylic acids are the sour molecules of chemistry, containing the distinctive carboxyl group . From the acetic acid in vinegar to the fatty acids in your cell membranes, these compounds are essential to life.
The general formula CₙH₂ₙ₊₁COOH covers everything from formic acid (ant venom) to complex fatty acids. Unlike other functional groups, the carboxyl group is always at the end of the chain, so you never need to number its position.
Neutralisation reactions are where carboxylic acids really shine. They react with alkalis to form salts and water, following the classic acid-base pattern. These reactions are crucial in everything from antacid tablets to soap making.
The carboxyl group is actually two functional groups merged together - a carbonyl and a hydroxyl . This combination creates unique properties that neither group has alone.
Daily Life: Every time you eat vinegar or citrus fruit, you're experiencing carboxylic acids in action!

Esters are chemistry's perfume makers - they're responsible for most fruity smells and flavours you encounter. They form through condensation reactions between carboxylic acids and alcohols, where water is eliminated to create the ester link .
Esterification is reversible, meaning esters can break back down into their parent alcohol and acid. This equilibrium is crucial in biological processes like fat digestion and metabolism.
Naming esters follows a simple two-step dance: change the alcohol name to end in "-yl" and the acid name to end in "-oate", then put alcohol first, acid second. So methanol + ethanoic acid becomes methyl ethanoate.
The general formula CₙH₂ₙO₂ applies to simple esters, though more complex ones exist. Esters are everywhere - in fruit flavourings, perfumes, and even the polyester in your clothes.
Smell Test: Next time you smell banana flavouring, you're detecting isoamyl acetate - a classic ester that tricks your nose!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Amy Neill
@amyneill
Time to dive into organic chemistry and discover how carbon compounds shape our world! This unit covers the main families of organic molecules, from simple hydrocarbons to complex alcohols and acids, showing you how to name them and understand their... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This is your gateway to understanding organic chemistry - the study of carbon-based compounds that make up everything from petrol to perfumes. You'll master the art of naming molecules and recognising patterns in their behaviour.
The key to success here is learning to spot the structural patterns that determine how molecules behave. Once you've cracked the code, you'll see chemistry everywhere around you.
Quick Tip: Keep the naming rules handy - they're your roadmap through this entire topic!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why petrol burns differently to cooking oil? It's all about homologous series - families of compounds that share similar structures and properties. Think of them as chemical cousins with the same general formula but different sizes.
The main families you need to know are alkanes (like methane), alkenes (like ethene), cycloalkanes, alcohols, and carboxylic acids. Each has its own general formula that acts like a mathematical recipe for building molecules.
When naming branched molecules, follow the four golden rules: find the longest chain, identify branches, number carbons to get the lowest numbers, then list branches alphabetically. Remember "McBride Educates People By Pretending He Has Oscars" for the carbon chain lengths.
Isomers are like molecular twins - same formula, different arrangement. They're crucial because even tiny structural changes can completely alter a compound's properties.
Memory Hook: Use the mnemonic to nail those carbon chain prefixes - it'll save you marks in exams!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's where chemistry gets exciting - saturated compounds are like fully packed suitcases (only single bonds), while unsaturated compounds have room for more (contain double bonds). This difference completely changes how they react.
The bromine test is your go-to method for spotting unsaturated compounds. Add bromine water and watch - if it goes from orange to colourless, you've got double bonds present. It's like a chemical detective test.
Addition reactions happen when small molecules crash into double bonds and stick there. Whether it's bromination, hydrogenation, chlorination, or hydration, the pattern is always the same - the double bond breaks and new atoms join the party.
Understanding these reactions isn't just academic - they're used to make everything from margarine (hydrogenation) to plastics (polymerisation).
Real World: Hydrogenation turns liquid oils into solid margarine - that's addition reactions in your kitchen!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Meet benzene - chemistry's rebel that breaks all the rules you've just learned. Despite looking like it has double bonds, benzene doesn't behave like other unsaturated compounds. It's got delocalised electrons that make it incredibly stable.
This stability means benzene won't react with bromine like other unsaturated compounds. Think of it as chemistry's equivalent of a perfectly balanced spinning top - it's happy as it is and doesn't want to change.
Phenol and phenyl groups are benzene's derivatives that pop up everywhere in organic chemistry. Phenol is benzene with an -OH group attached, making it useful for everything from antiseptics to plastics.
The key insight here is that aromatic compounds march to their own beat - they prefer substitution reactions over addition reactions because they want to keep their stable ring structure.
Key Point: Benzene's stability comes from electron delocalisation - imagine electrons spread like butter across the entire ring rather than stuck between specific atoms.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Alcohols are the party molecules of organic chemistry - they contain the hydroxyl group that makes them incredibly versatile. From the ethanol in hand sanitiser to the glycerol in soap, alcohols are everywhere.
The general formula CₙH₂ₙ₊₁OH is your key to identifying any alcohol. But chemistry loves variety, so you'll also meet diols and triols like glycerol.
Glycerol is particularly important - it's the backbone of fats and oils in your body. Understanding its structure helps explain why fats behave the way they do.
The position of that -OH group matters enormously. Moving it just one carbon along the chain can change a molecule from a useful fuel additive to a toxic poison.
Fun Fact: Glycerol is what makes soap slippery and helps keep your skin moisturised!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Not all alcohols are created equal - they come in three distinct types based on where that hydroxyl group attaches. Primary alcohols have the -OH attached to a carbon with only one other carbon neighbour. Think of them as the end-of-the-line molecules.
Secondary alcohols are the middle children - their -OH sits on a carbon with two carbon neighbours. These often make great solvents and appear in many household products.
Tertiary alcohols are the crowded ones - three carbon atoms surround the carbon bearing the -OH group. This crowding makes them behave very differently in reactions, often making them more resistant to oxidation.
Why does this matter? Each type reacts differently when you try to oxidise them, producing different products. Primary alcohols can become carboxylic acids, secondary become ketones, but tertiary alcohols stubbornly refuse to oxidise under normal conditions.
Memory Trick: Primary = one neighbour, Secondary = two neighbours, Tertiary = three neighbours. Simple!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why alcohols have such different properties from their alkane cousins? It all comes down to that polar hydroxyl group and its ability to form hydrogen bonds. These are like molecular handshakes that hold molecules together more tightly.
Melting and boiling points of alcohols are much higher than equivalent alkanes because hydrogen bonds are stronger than the weak London dispersion forces in alkanes. Breaking hydrogen bonds requires more energy, hence higher temperatures.
This explains why ethanol (alcohol) boils at 78°C while ethane boils at -89°C, despite having similar molecular weights. The hydrogen bonding makes all the difference.
As alcohol molecules get larger, they become less polar overall. The hydrocarbon chain grows while the -OH group stays the same size, gradually changing the molecule's personality from polar to non-polar.
Key Insight: Hydrogen bonds are the strongest intermolecular forces - they're what make alcohols so different from hydrocarbons.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Solubility in water follows a clear pattern - short-chain alcohols dissolve brilliantly because they can form hydrogen bonds with water molecules. But as the carbon chain grows longer, the non-polar hydrocarbon part takes over and solubility drops dramatically.
Ethanol mixes with water in any proportion (that's why alcoholic drinks exist!), but try dissolving octanol and you'll be waiting forever. The long hydrocarbon chain makes it behave more like oil than alcohol.
This creates fascinating trends as you move up the alcohol series. Flammability decreases, molecular mass increases, solubility in water drops, and melting/boiling points rise steadily.
Understanding these trends helps explain why methanol is used as a fuel, ethanol for drinks and antiseptics, and longer alcohols for industrial solvents and lubricants.
Real World: This is why vodka (40% ethanol) mixes with water, but cooking oil doesn't - it's all about polarity and hydrogen bonding!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Carboxylic acids are the sour molecules of chemistry, containing the distinctive carboxyl group . From the acetic acid in vinegar to the fatty acids in your cell membranes, these compounds are essential to life.
The general formula CₙH₂ₙ₊₁COOH covers everything from formic acid (ant venom) to complex fatty acids. Unlike other functional groups, the carboxyl group is always at the end of the chain, so you never need to number its position.
Neutralisation reactions are where carboxylic acids really shine. They react with alkalis to form salts and water, following the classic acid-base pattern. These reactions are crucial in everything from antacid tablets to soap making.
The carboxyl group is actually two functional groups merged together - a carbonyl and a hydroxyl . This combination creates unique properties that neither group has alone.
Daily Life: Every time you eat vinegar or citrus fruit, you're experiencing carboxylic acids in action!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Esters are chemistry's perfume makers - they're responsible for most fruity smells and flavours you encounter. They form through condensation reactions between carboxylic acids and alcohols, where water is eliminated to create the ester link .
Esterification is reversible, meaning esters can break back down into their parent alcohol and acid. This equilibrium is crucial in biological processes like fat digestion and metabolism.
Naming esters follows a simple two-step dance: change the alcohol name to end in "-yl" and the acid name to end in "-oate", then put alcohol first, acid second. So methanol + ethanoic acid becomes methyl ethanoate.
The general formula CₙH₂ₙO₂ applies to simple esters, though more complex ones exist. Esters are everywhere - in fruit flavourings, perfumes, and even the polyester in your clothes.
Smell Test: Next time you smell banana flavouring, you're detecting isoamyl acetate - a classic ester that tricks your nose!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
9
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user