Chemistry is all about understanding how atoms interact and change... Show more
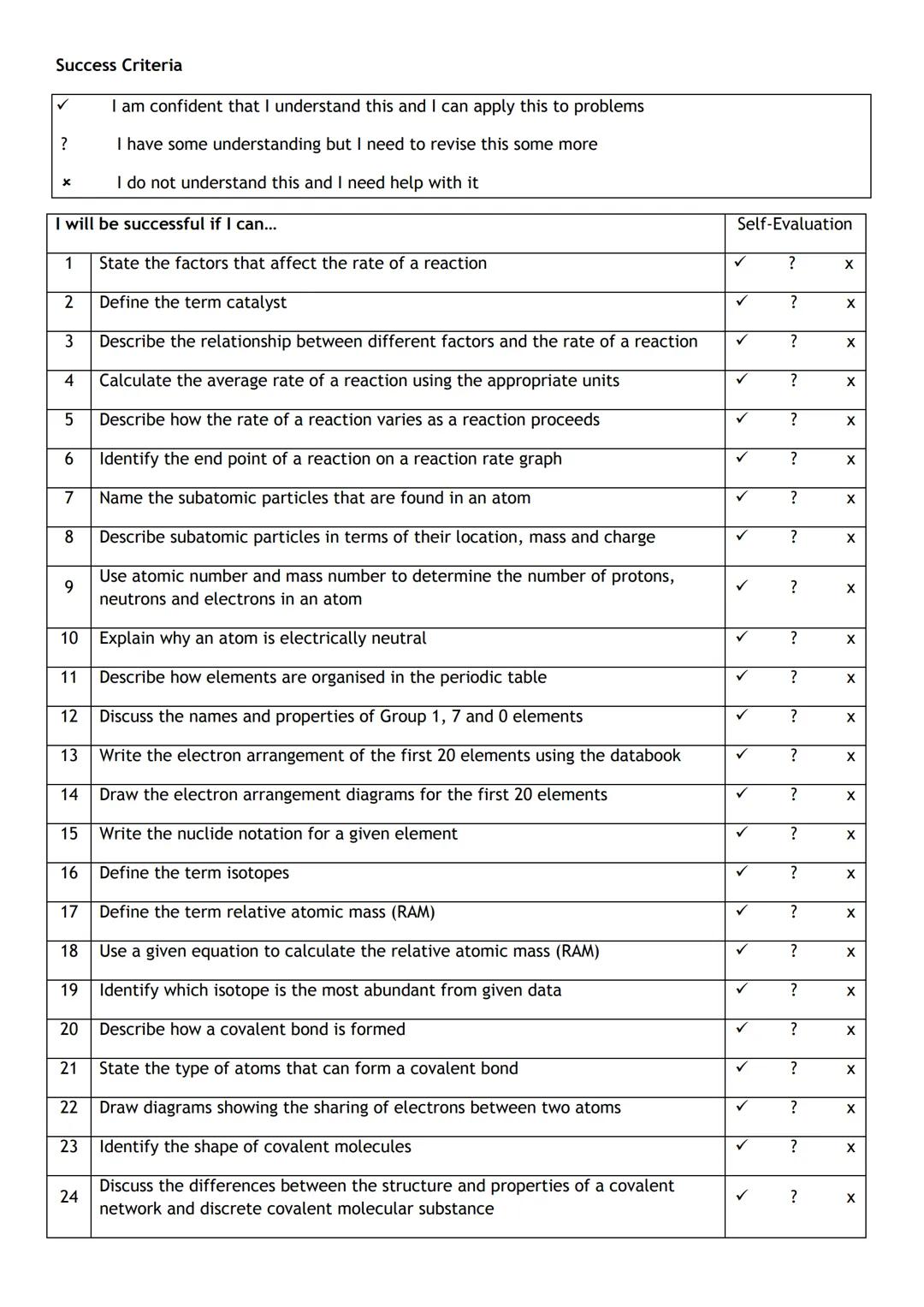
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
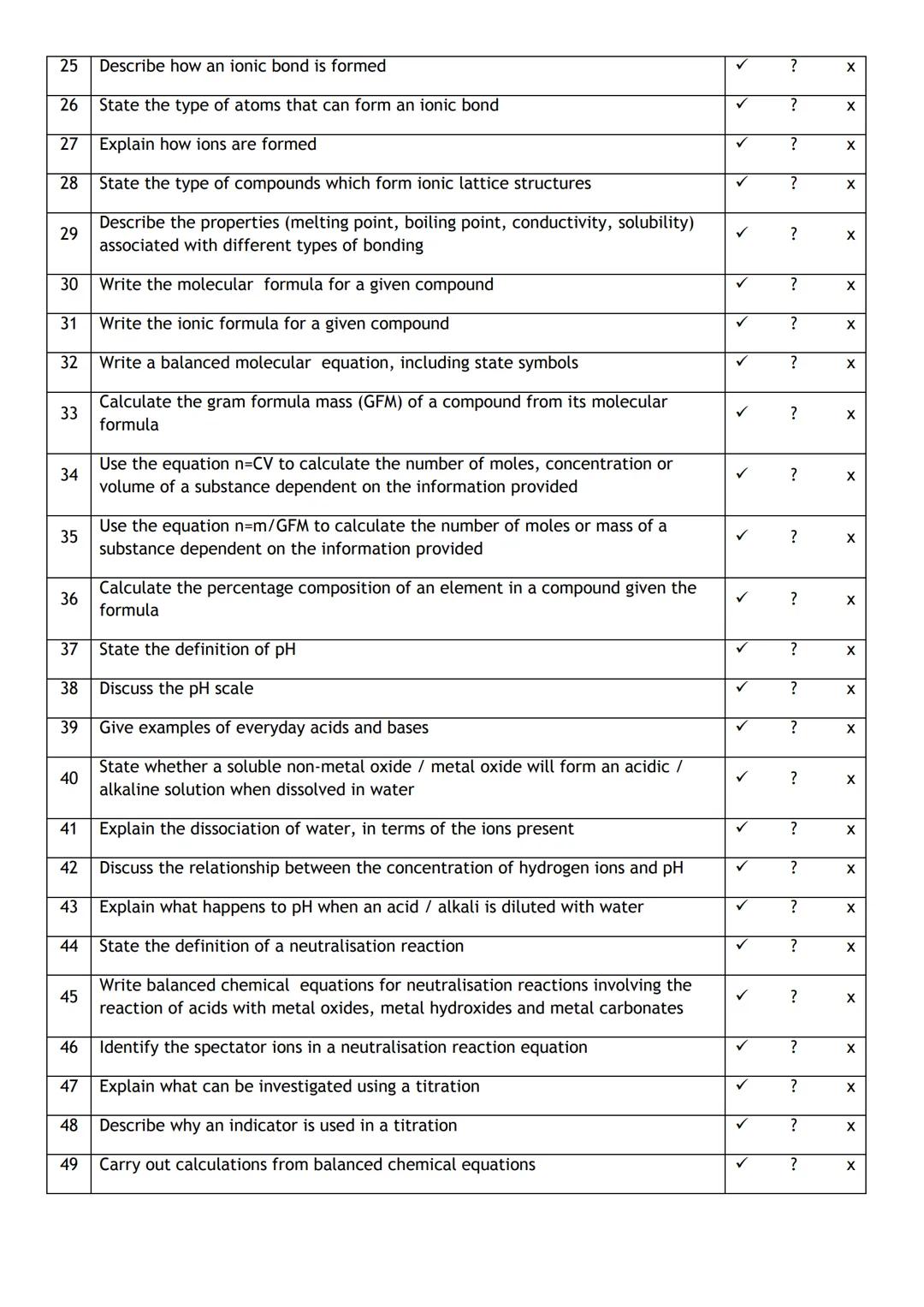
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
300
•
4 Feb 2026
•
Holly Hutchison
@hollyhutchison
Chemistry is all about understanding how atoms interact and change... Show more



You've got a brilliant roadmap here that covers all the essential chemistry concepts you'll need to master. Think of this as your personal progress tracker - it's designed to help you identify exactly where you stand with each topic.
The checklist covers four major areas: reaction rates, atomic structure, chemical bonding, and calculations. Each skill builds on the previous ones, so don't worry if some areas feel trickier than others - that's completely normal!
Use the tick, question mark, and cross system honestly. Being realistic about what you understand now will help you focus your revision time where it's needed most.
Top Tip: Come back to this checklist regularly as you study - watching those crosses turn into ticks is incredibly motivating!
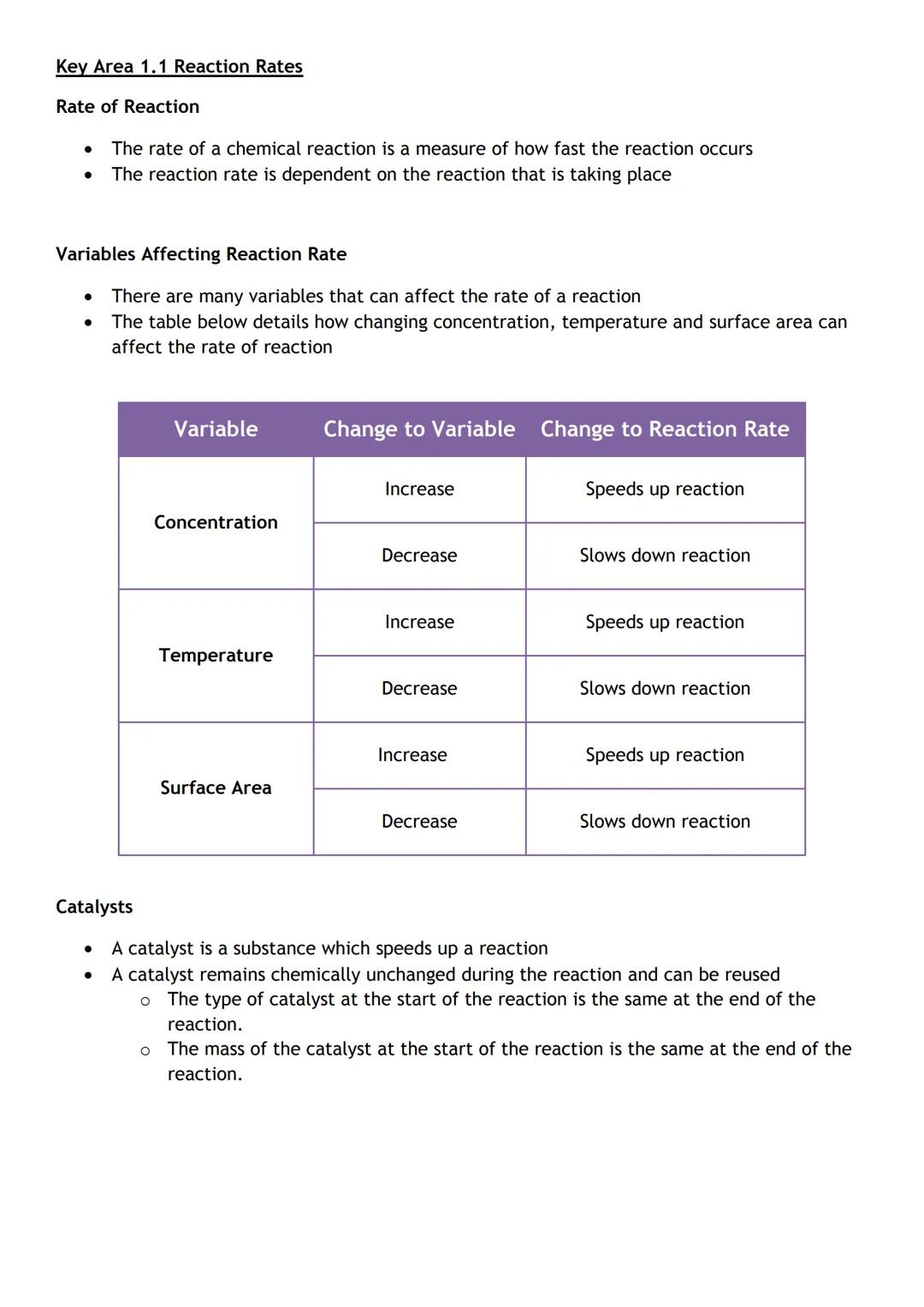
Ever wondered why some chemical reactions happen instantly whilst others take ages? Reaction rate measures how quickly reactants disappear and products form - and you can actually control this speed.
Three main factors affect how fast reactions occur. Concentration matters because more particles in a smaller space means more collisions. Temperature speeds things up because particles move faster and collide more energetically. Surface area increases reaction rate because there's more contact between reactants.
Catalysts are like chemical cheat codes - they speed up reactions without getting used up themselves. They remain completely unchanged at the end, so you can use them again and again.
Real-world connection: This explains why food cooks faster at higher temperatures and why powdered medicine works quicker than tablets!
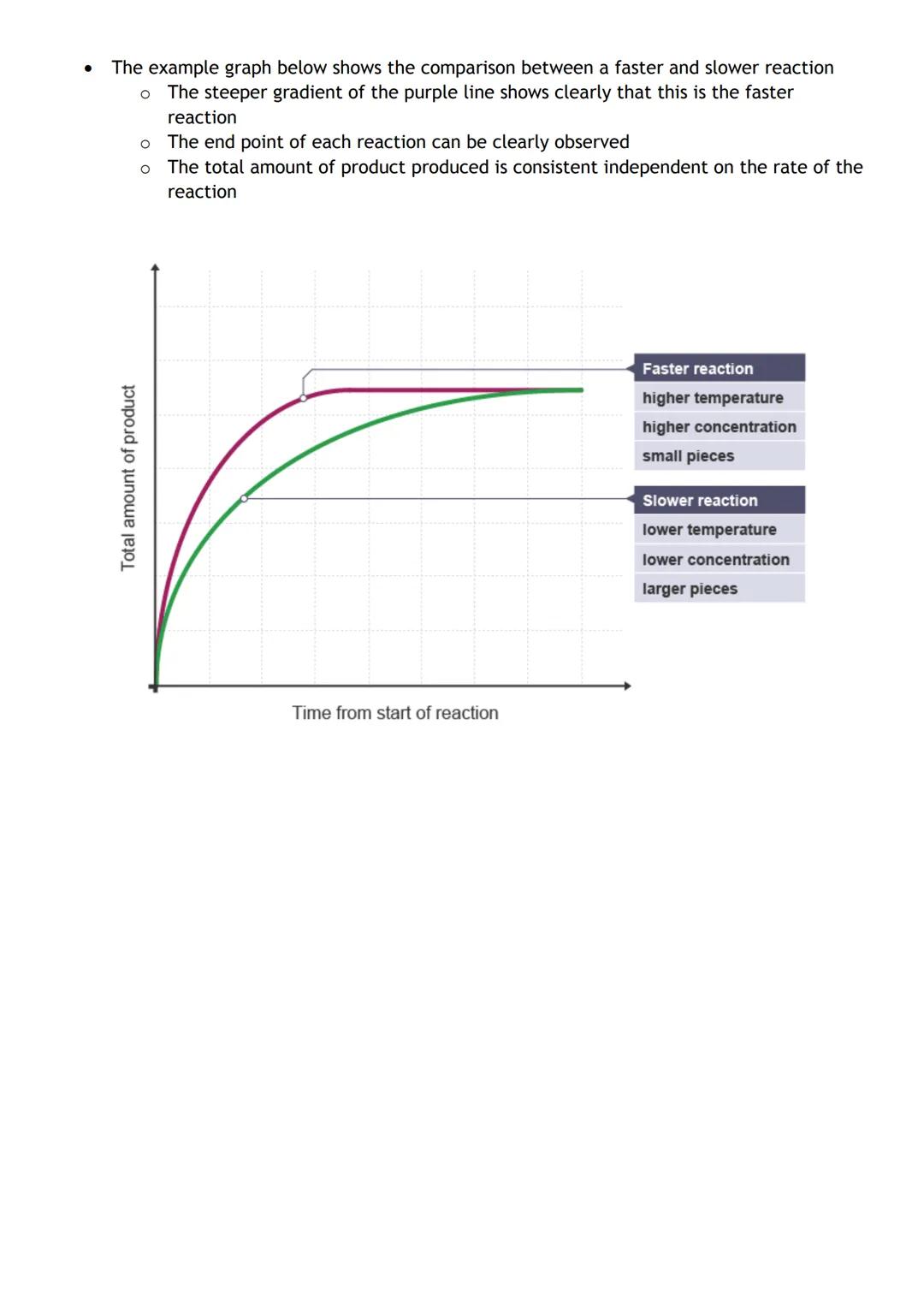
You can monitor reactions by tracking changes in mass, volume, or concentration over time. The key equation you need is: Average rate = Change in measurable quantity ÷ Change in time.
Rate graphs tell the whole story of a reaction. The steeper the line, the faster the reaction. When the line goes horizontal, the reaction has stopped completely. You can read off how much product formed and exactly when the reaction finished.
Comparing multiple experiments on the same graph shows you which conditions make reactions faster. The purple line beating the blue line? That's your higher temperature or increased concentration in action.
Units matter! If you're measuring mass, your rate will be in g/s (grams per second). For volume changes, use cm³/s (cubic centimetres per second).
Exam tip: Always check your units match the measurement you're tracking - it's an easy way to pick up marks!
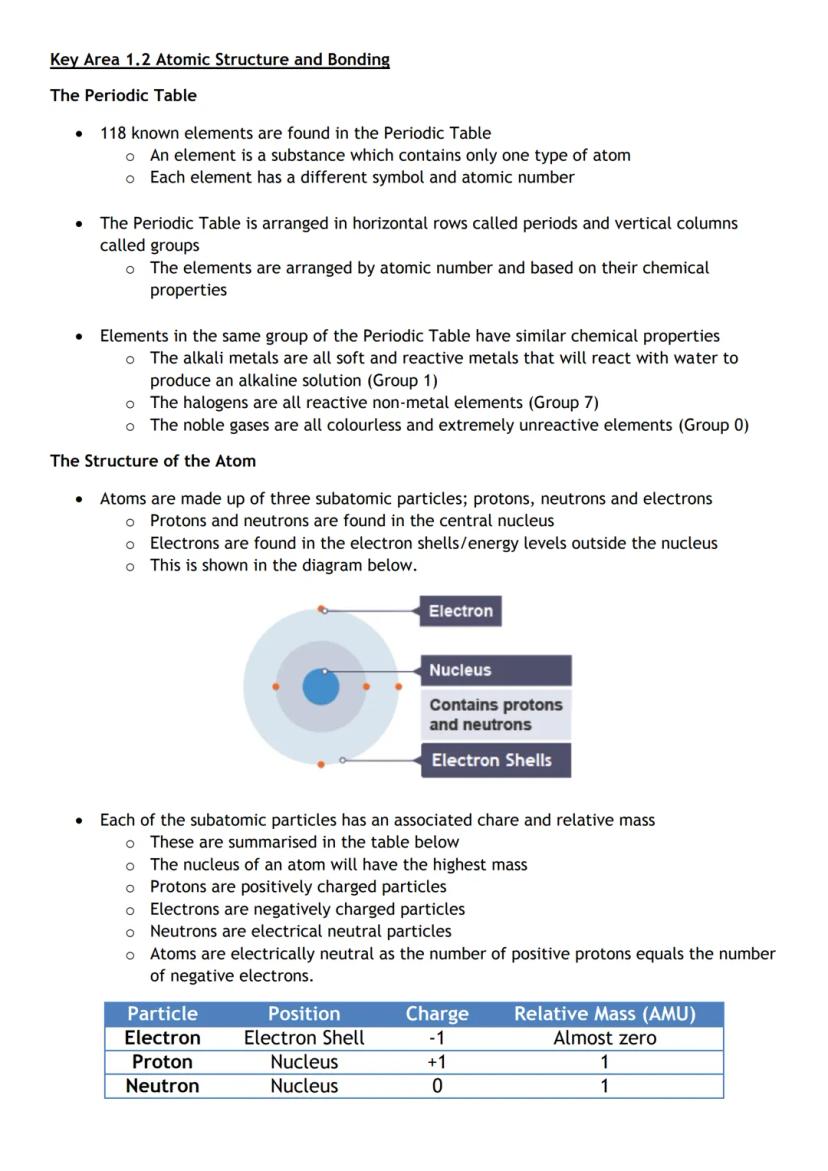
The periodic table organises all 118 known elements by atomic number and groups elements with similar properties together. It's like a massive family tree where relatives share characteristics.
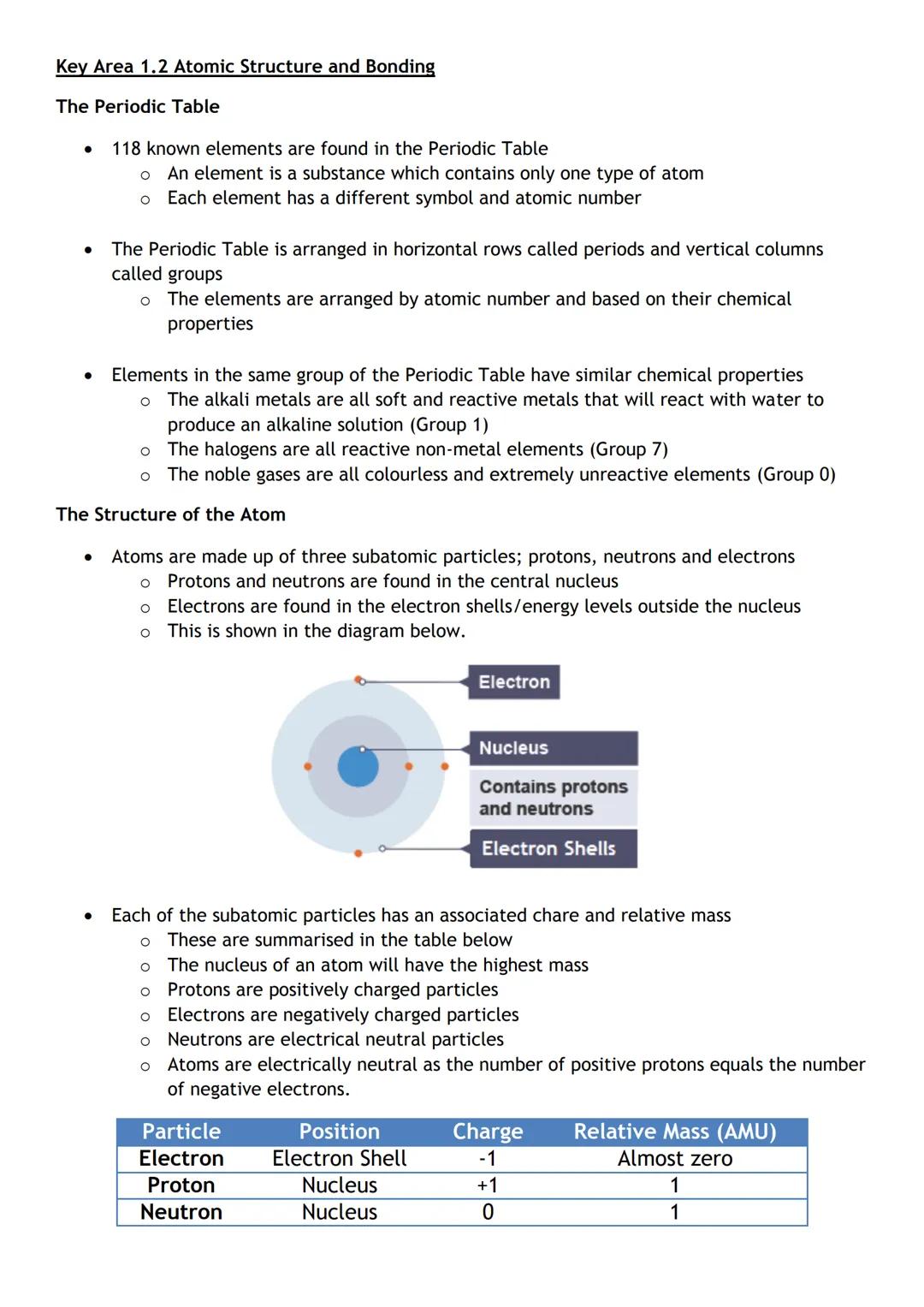
Atoms contain three types of particles. Protons and neutrons live in the central nucleus. Electrons (negative charge, almost no mass) whizz around in shells outside the nucleus.
Here's the crucial bit: atoms are electrically neutral because they have equal numbers of protons and electrons. The positive and negative charges cancel each other out perfectly.
Groups in the periodic table have predictable properties. Group 1 metals are soft and reactive. Group 7 halogens are reactive non-metals. Group 0 noble gases barely react with anything.
Memory trick: Think of the atom like a football stadium - the tiny nucleus is the centre circle, whilst electrons fill the stands around it!

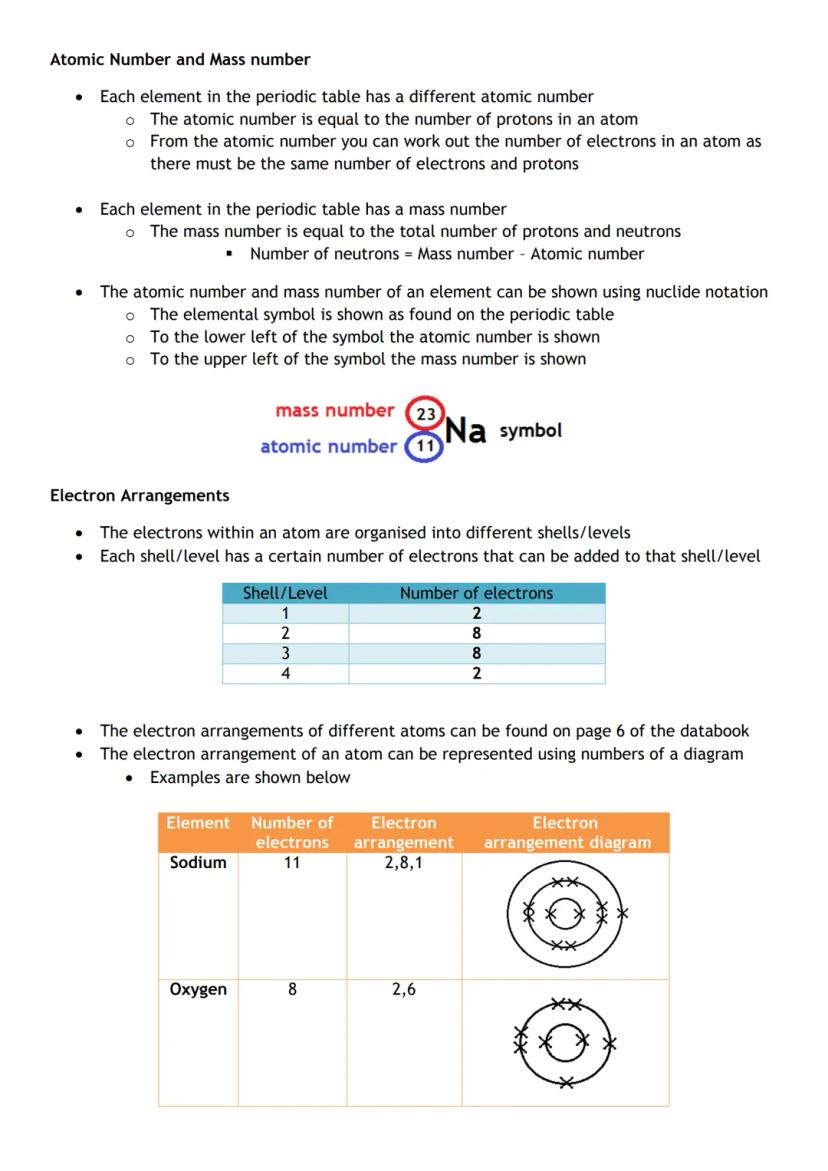
Every element has a unique atomic number - that's the number of protons in its atoms. Since atoms are neutral, the atomic number also tells you the number of electrons.
Mass number equals protons plus neutrons. To find neutrons: Mass number - Atomic number = Number of neutrons. Nuclide notation shows this clearly with mass number top-left and atomic number bottom-left of the element symbol.
Electron arrangements follow specific rules. The first shell holds 2 electrons maximum, the second holds 8, the third holds 8, and the fourth holds 2. You'll find these arrangements listed in your databook for the first 20 elements.
Sodium (11 electrons) arranges as 2,8,1. Oxygen (8 electrons) arranges as 2,6. These arrangements determine how elements behave chemically.
Study smart: Don't memorise all the electron arrangements - learn the shell capacity rules and you can work them out!
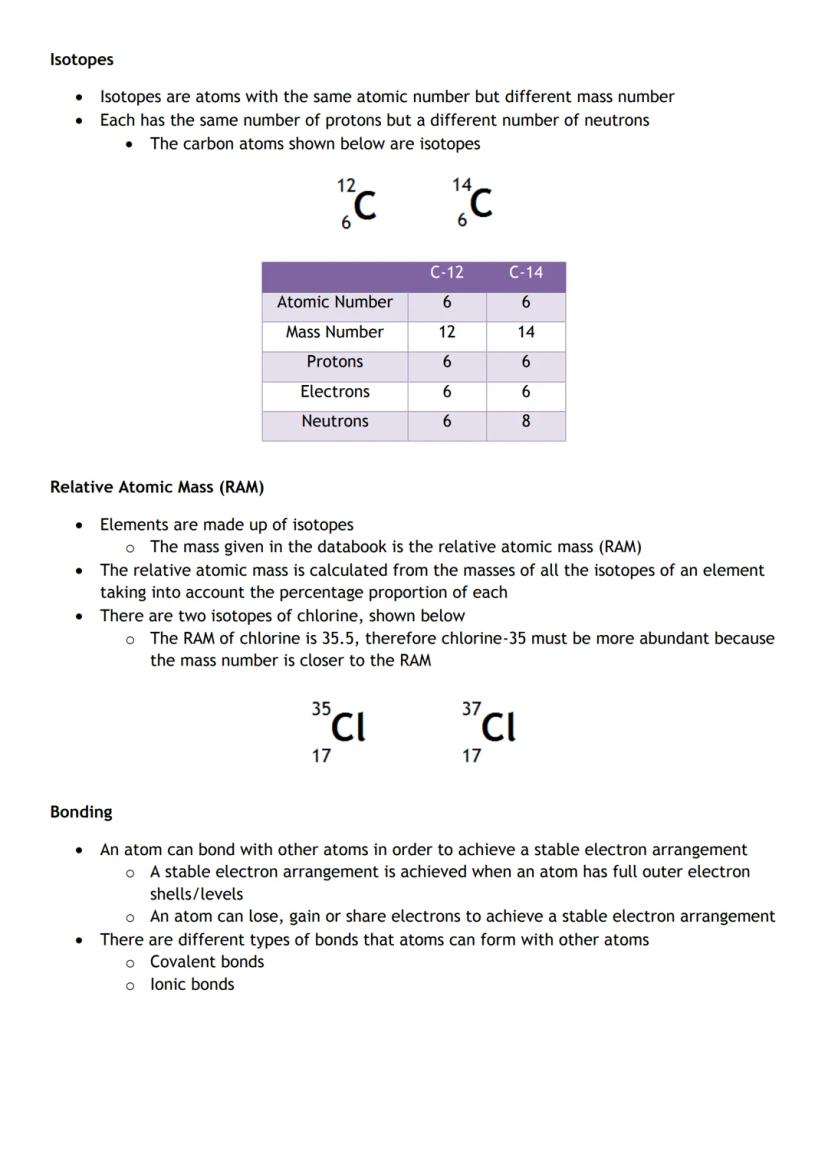
Isotopes are atoms of the same element with different numbers of neutrons. Carbon-12 and Carbon-14 both have 6 protons but different mass numbers due to extra neutrons.
Relative atomic mass (RAM) accounts for all isotopes of an element. If chlorine's RAM is 35.5, then chlorine-35 must be more common than chlorine-37 (because the average is closer to 35).
Atoms bond to achieve stable electron arrangements - basically, they want full outer shells. They can lose, gain, or share electrons to get there, creating two main bond types: covalent and ionic.
Think of bonding like atoms trying to feel complete. They'll do whatever it takes to fill their outer electron shells!
Key insight: The drive for stability explains all chemical bonding - atoms are just trying to achieve their most comfortable state.
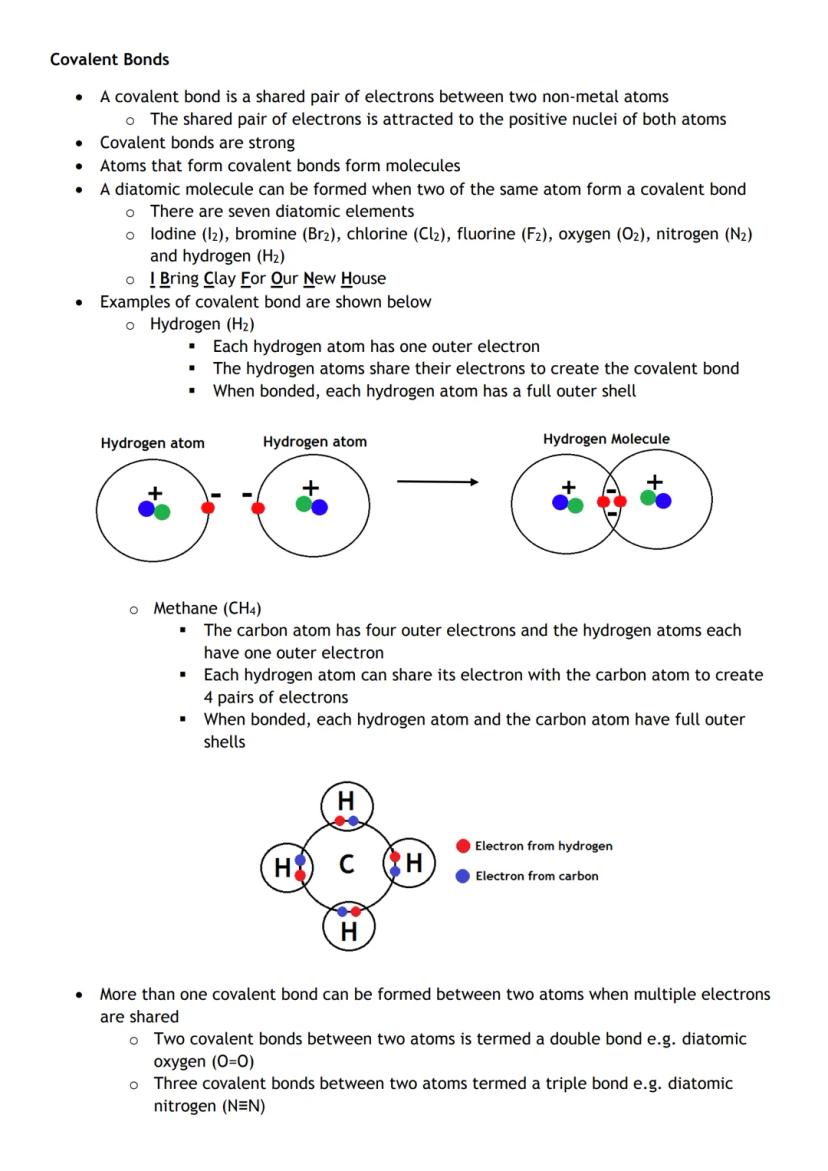
Covalent bonds form when two non-metal atoms share electrons. It's like a chemical handshake where both atoms benefit from the shared pair.
The seven diatomic elements (I Bring Clay For Our New House: I₂, Br₂, Cl₂, F₂, O₂, N₂, H₂) naturally exist as molecules with covalent bonds between identical atoms.
In hydrogen gas (H₂), each hydrogen shares its single electron, giving both atoms a full outer shell. In methane (CH₄), carbon shares electrons with four hydrogens, satisfying everyone's needs.
Double bonds and triple bonds (like N≡N) form when atoms share multiple electron pairs. More shared electrons mean stronger bonds.
Visual learner tip: Draw out the electron sharing diagrams - seeing how electrons pair up makes covalent bonding click instantly!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Holly Hutchison
@hollyhutchison
Chemistry is all about understanding how atoms interact and change - and it's way more relevant to your daily life than you might think! This National 5 Chemistry guide breaks down everything from why reactions happen at different speeds to... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
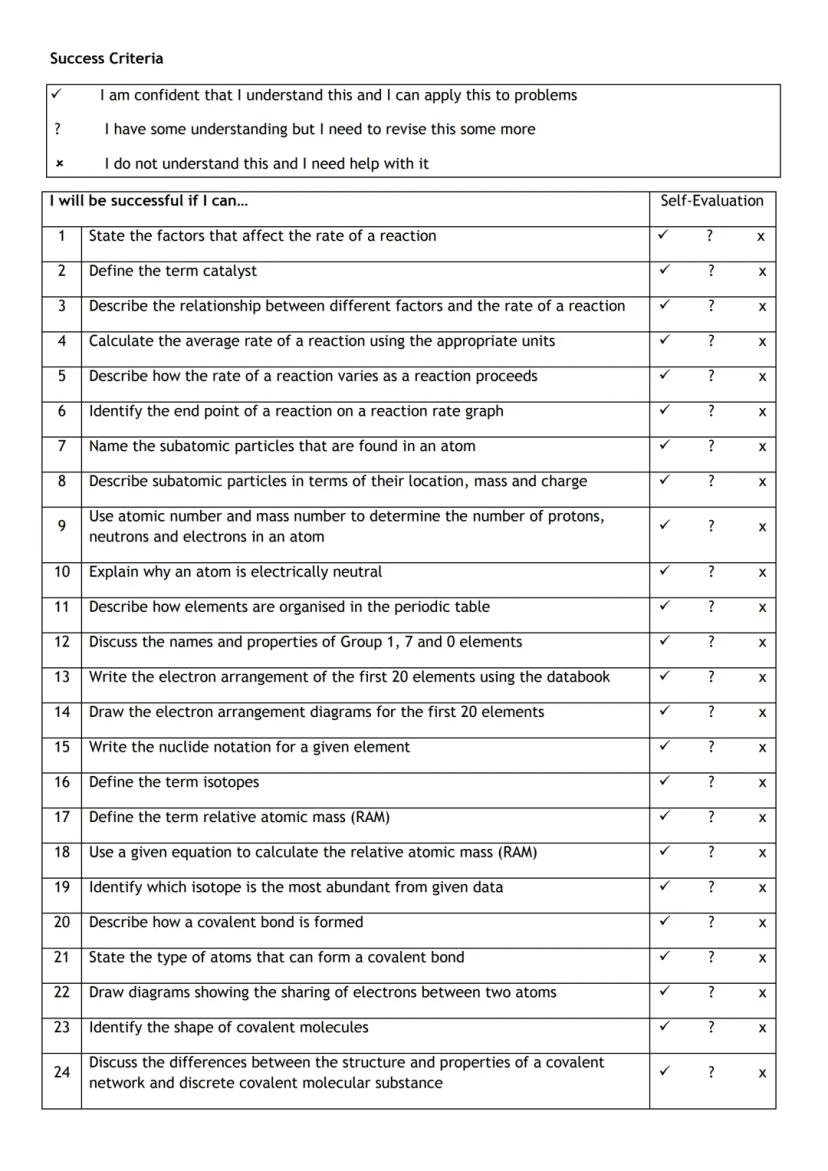
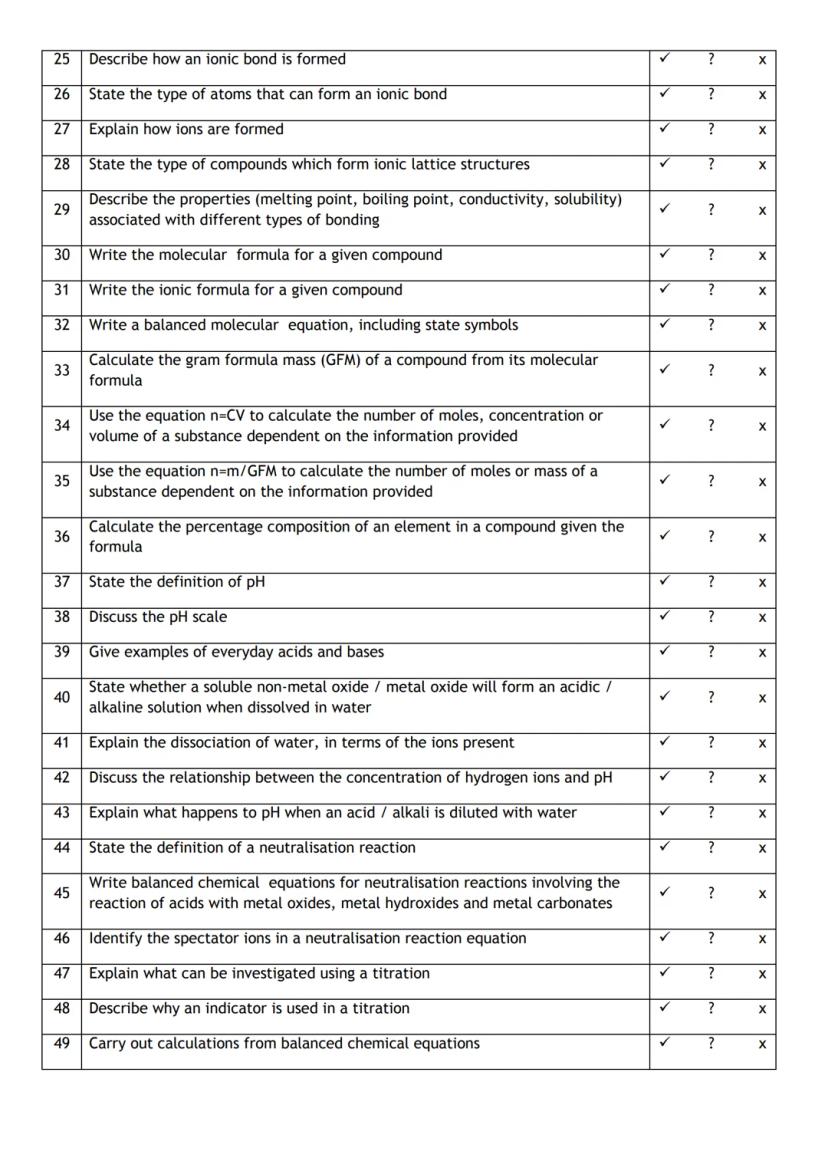
You've got a brilliant roadmap here that covers all the essential chemistry concepts you'll need to master. Think of this as your personal progress tracker - it's designed to help you identify exactly where you stand with each topic.
The checklist covers four major areas: reaction rates, atomic structure, chemical bonding, and calculations. Each skill builds on the previous ones, so don't worry if some areas feel trickier than others - that's completely normal!
Use the tick, question mark, and cross system honestly. Being realistic about what you understand now will help you focus your revision time where it's needed most.
Top Tip: Come back to this checklist regularly as you study - watching those crosses turn into ticks is incredibly motivating!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
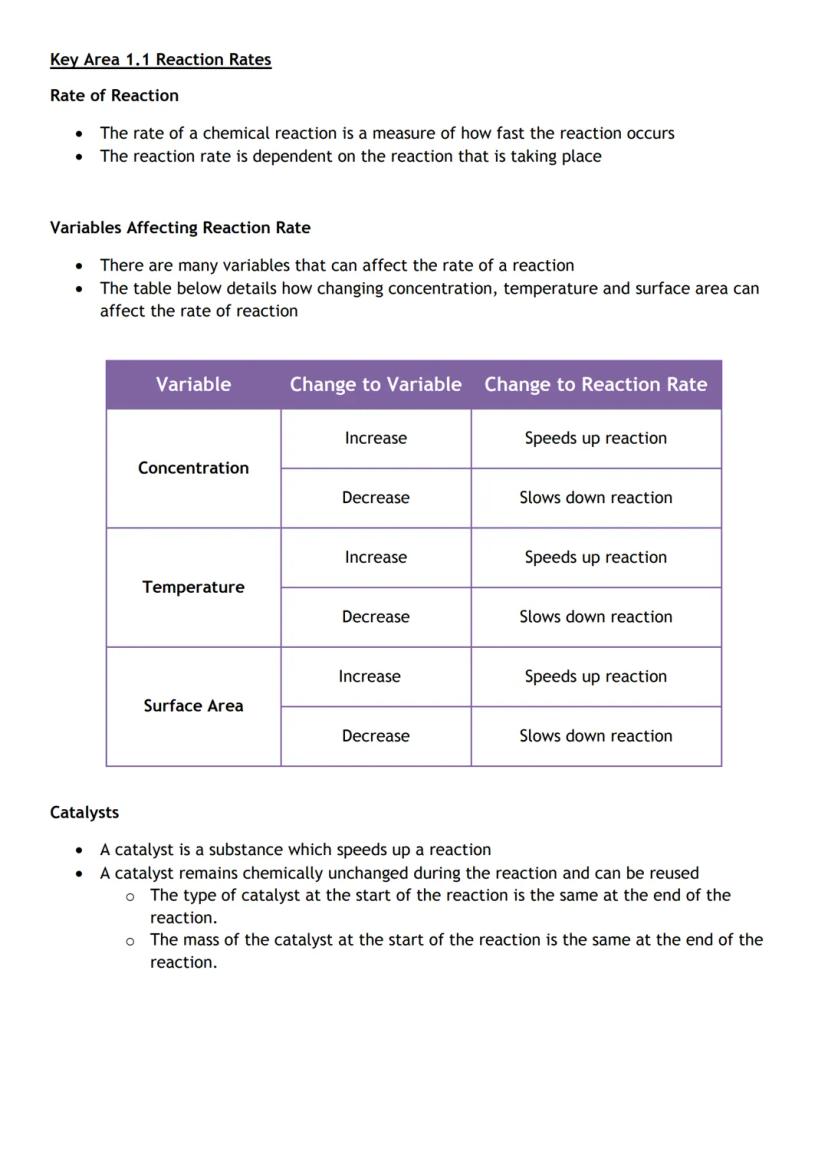
Ever wondered why some chemical reactions happen instantly whilst others take ages? Reaction rate measures how quickly reactants disappear and products form - and you can actually control this speed.
Three main factors affect how fast reactions occur. Concentration matters because more particles in a smaller space means more collisions. Temperature speeds things up because particles move faster and collide more energetically. Surface area increases reaction rate because there's more contact between reactants.
Catalysts are like chemical cheat codes - they speed up reactions without getting used up themselves. They remain completely unchanged at the end, so you can use them again and again.
Real-world connection: This explains why food cooks faster at higher temperatures and why powdered medicine works quicker than tablets!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
You can monitor reactions by tracking changes in mass, volume, or concentration over time. The key equation you need is: Average rate = Change in measurable quantity ÷ Change in time.
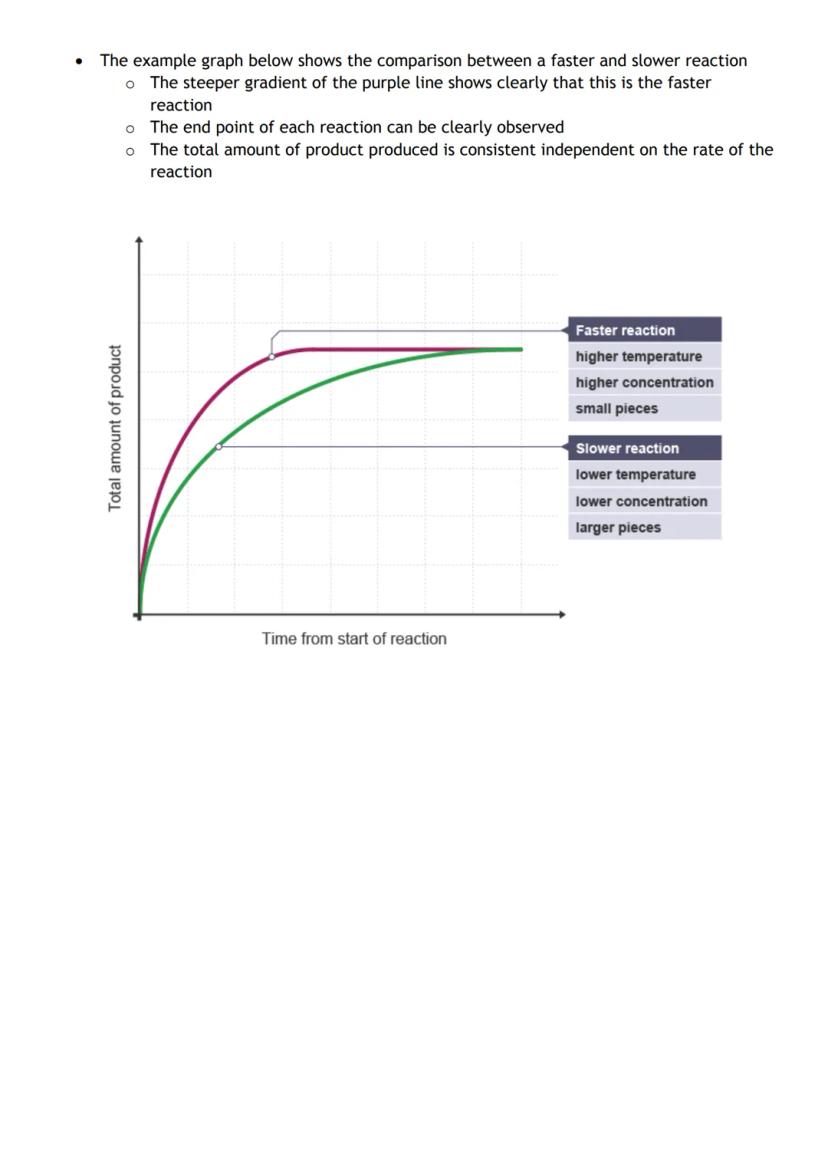
Rate graphs tell the whole story of a reaction. The steeper the line, the faster the reaction. When the line goes horizontal, the reaction has stopped completely. You can read off how much product formed and exactly when the reaction finished.
Comparing multiple experiments on the same graph shows you which conditions make reactions faster. The purple line beating the blue line? That's your higher temperature or increased concentration in action.
Units matter! If you're measuring mass, your rate will be in g/s (grams per second). For volume changes, use cm³/s (cubic centimetres per second).
Exam tip: Always check your units match the measurement you're tracking - it's an easy way to pick up marks!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The periodic table organises all 118 known elements by atomic number and groups elements with similar properties together. It's like a massive family tree where relatives share characteristics.
Atoms contain three types of particles. Protons and neutrons live in the central nucleus. Electrons (negative charge, almost no mass) whizz around in shells outside the nucleus.
Here's the crucial bit: atoms are electrically neutral because they have equal numbers of protons and electrons. The positive and negative charges cancel each other out perfectly.
Groups in the periodic table have predictable properties. Group 1 metals are soft and reactive. Group 7 halogens are reactive non-metals. Group 0 noble gases barely react with anything.
Memory trick: Think of the atom like a football stadium - the tiny nucleus is the centre circle, whilst electrons fill the stands around it!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Every element has a unique atomic number - that's the number of protons in its atoms. Since atoms are neutral, the atomic number also tells you the number of electrons.
Mass number equals protons plus neutrons. To find neutrons: Mass number - Atomic number = Number of neutrons. Nuclide notation shows this clearly with mass number top-left and atomic number bottom-left of the element symbol.
Electron arrangements follow specific rules. The first shell holds 2 electrons maximum, the second holds 8, the third holds 8, and the fourth holds 2. You'll find these arrangements listed in your databook for the first 20 elements.
Sodium (11 electrons) arranges as 2,8,1. Oxygen (8 electrons) arranges as 2,6. These arrangements determine how elements behave chemically.
Study smart: Don't memorise all the electron arrangements - learn the shell capacity rules and you can work them out!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Isotopes are atoms of the same element with different numbers of neutrons. Carbon-12 and Carbon-14 both have 6 protons but different mass numbers due to extra neutrons.
Relative atomic mass (RAM) accounts for all isotopes of an element. If chlorine's RAM is 35.5, then chlorine-35 must be more common than chlorine-37 (because the average is closer to 35).
Atoms bond to achieve stable electron arrangements - basically, they want full outer shells. They can lose, gain, or share electrons to get there, creating two main bond types: covalent and ionic.
Think of bonding like atoms trying to feel complete. They'll do whatever it takes to fill their outer electron shells!
Key insight: The drive for stability explains all chemical bonding - atoms are just trying to achieve their most comfortable state.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Covalent bonds form when two non-metal atoms share electrons. It's like a chemical handshake where both atoms benefit from the shared pair.
The seven diatomic elements (I Bring Clay For Our New House: I₂, Br₂, Cl₂, F₂, O₂, N₂, H₂) naturally exist as molecules with covalent bonds between identical atoms.
In hydrogen gas (H₂), each hydrogen shares its single electron, giving both atoms a full outer shell. In methane (CH₄), carbon shares electrons with four hydrogens, satisfying everyone's needs.
Double bonds and triple bonds (like N≡N) form when atoms share multiple electron pairs. More shared electrons mean stronger bonds.
Visual learner tip: Draw out the electron sharing diagrams - seeing how electrons pair up makes covalent bonding click instantly!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
9
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user