Chemistry is everywhere around us - from why food cooks... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
1,266
•
8 Feb 2026
•
Amy Neill
@amyneill
Chemistry is everywhere around us - from why food cooks... Show more





You're about to dive into one of the most exciting parts of chemistry! This unit covers everything from reaction rates to atomic structure - basically, how fast things happen and why they happen at all.
Think of it like learning the rules of a game you've been watching your whole life. Once you understand these concepts, you'll start noticing chemistry everywhere - from cooking dinner to understanding why batteries work.
Quick Tip: These topics build on each other, so mastering the basics early will make everything else click into place much easier.

Ever wondered why chips cook faster than whole potatoes? It's all about reaction rates! Four main factors control how fast reactions happen, and once you understand them, you can predict and control chemical reactions.
Temperature is probably the most obvious one. Higher temperatures mean particles move faster, leading to more collisions and quicker reactions. That's why your food lasts ages in the fridge but goes off quickly in a warm room.
Concentration works like a busy corridor - more particles in the same space means more bumping into each other. A 1.0 mol/L acid will react way faster than a 0.1 mol/L acid because there are simply more reactive particles present.
Memory Trick: Think of students moving between classes - more students in a corridor means more collisions!

Here's where things get interesting! Particle size affects reaction rate because smaller particles have a larger surface area. Sugar granules dissolve faster than sugar cubes for exactly this reason - more surface area means more contact with water.
Catalysts are like that friend who stirs up drama but never gets in trouble themselves. They speed up reactions without being used up in the process. A catalyst provides an alternative pathway for the reaction that requires less energy.
The brilliant thing about catalysts is they remain unchanged at the end. Think of a teabag - it helps make your tea but comes out still looking like a teabag!
Real-world Connection: Car catalytic converters use this principle to break down harmful exhaust gases without being consumed themselves.
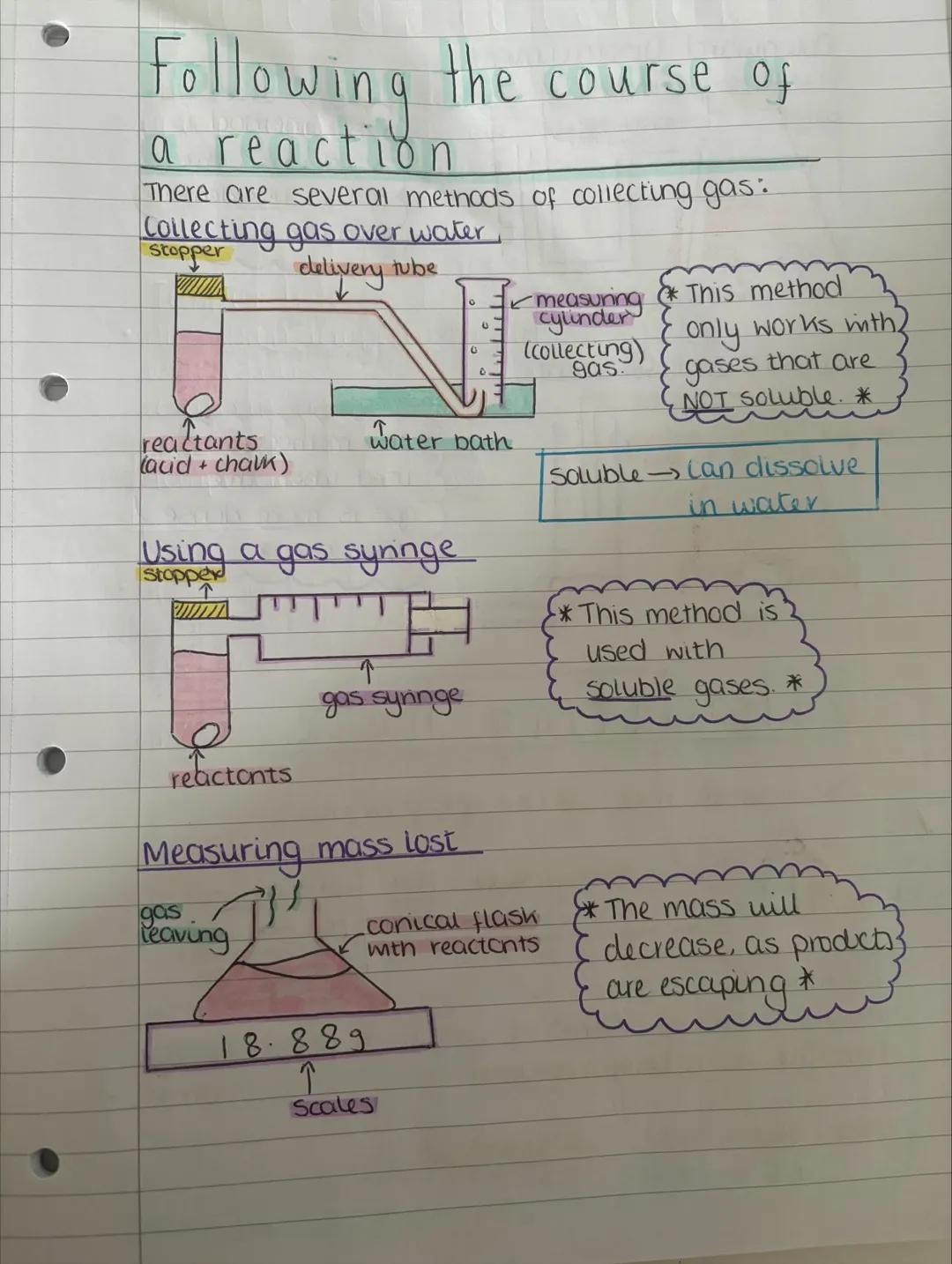
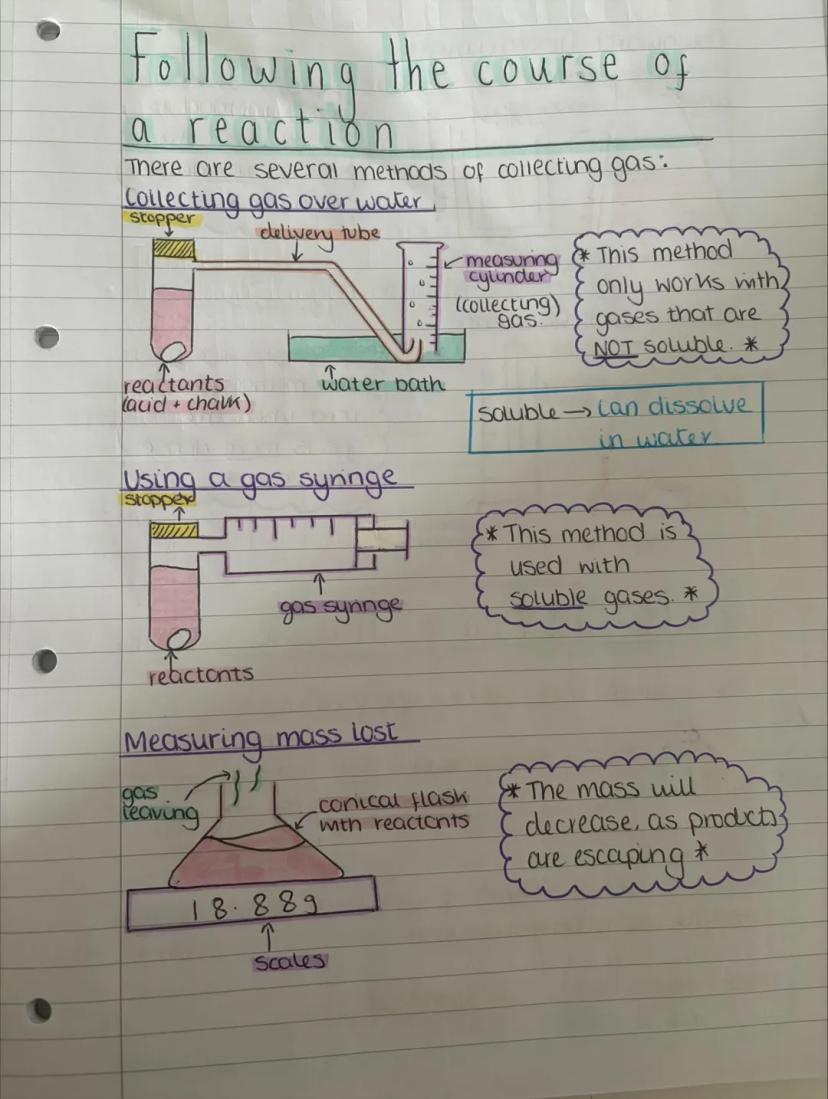
Now you can actually measure how fast reactions happen! There are several clever ways to collect and measure gases produced during reactions, each suited to different types of gases.
Collecting gas over water only works with gases that won't dissolve in water. You simply let the gas push water out of an upturned measuring cylinder - dead simple but effective.
For soluble gases, you'll need a gas syringe instead. This method captures gases that would otherwise dissolve and disappear into the water. You can also measure mass lost by weighing the reaction flask as gases escape - the mass decreases as products leave the system.
Lab Success: Always check if your gas is soluble in water before choosing your collection method - it'll save you from getting rubbish results!
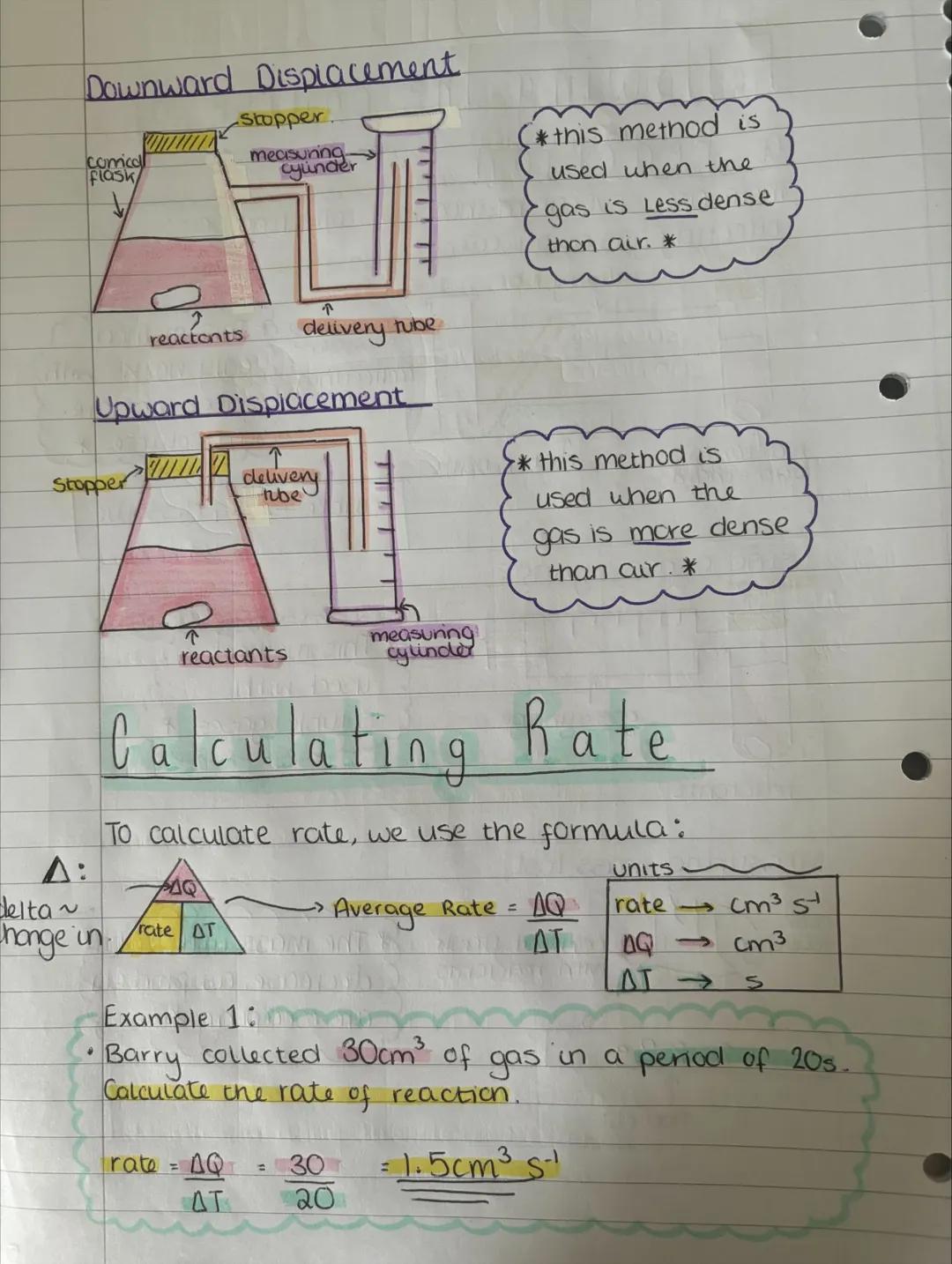
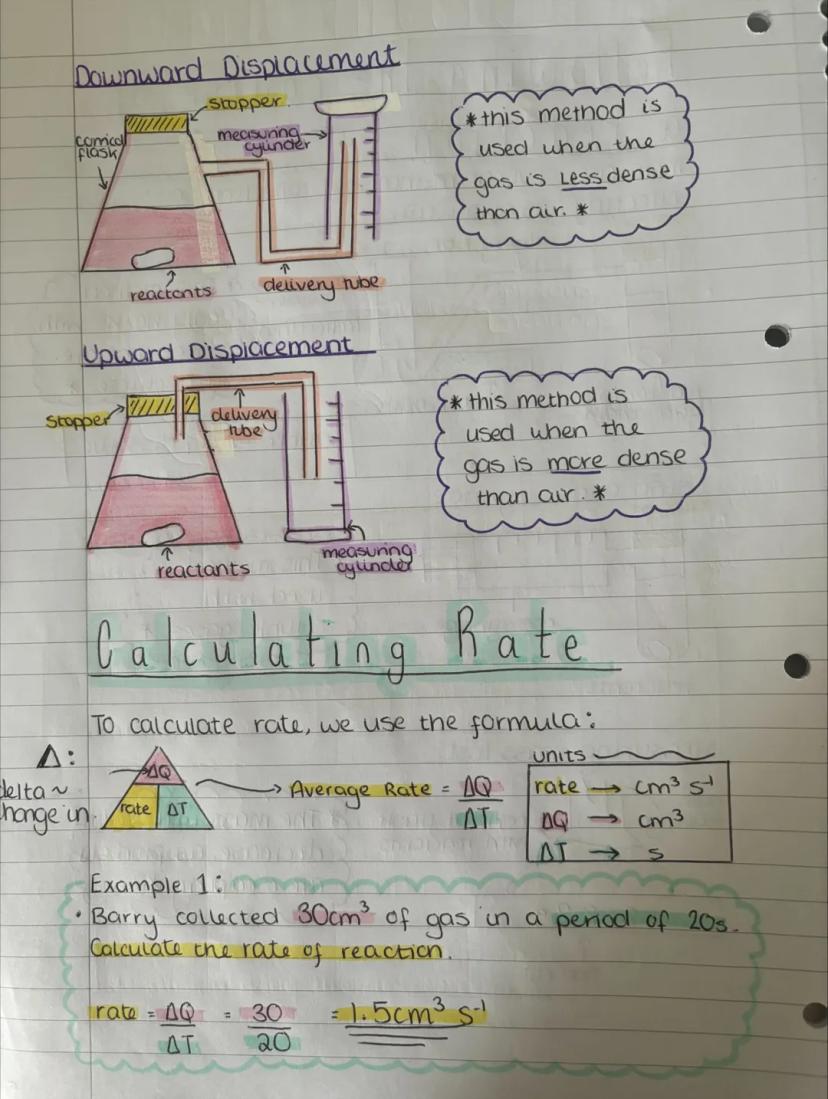
Displacement methods depend on whether your gas is lighter or heavier than air. Upward displacement works for gases less dense than air, while downward displacement suits gases more dense than air.
Calculating reaction rates uses a straightforward formula: Rate = ΔQ/ΔT. The Greek letter delta (Δ) just means "change in", so you're finding change in quantity over change in time.
For example, if Barry collected 30 cm³ of gas in 20 seconds, his rate would be 30/20 = 1.5 cm³ s⁻¹. Simple maths, but it tells you exactly how fast the reaction is happening!
Exam Tip: Always include units in your rate calculations - typically cm³ s⁻¹ for gas collection experiments.
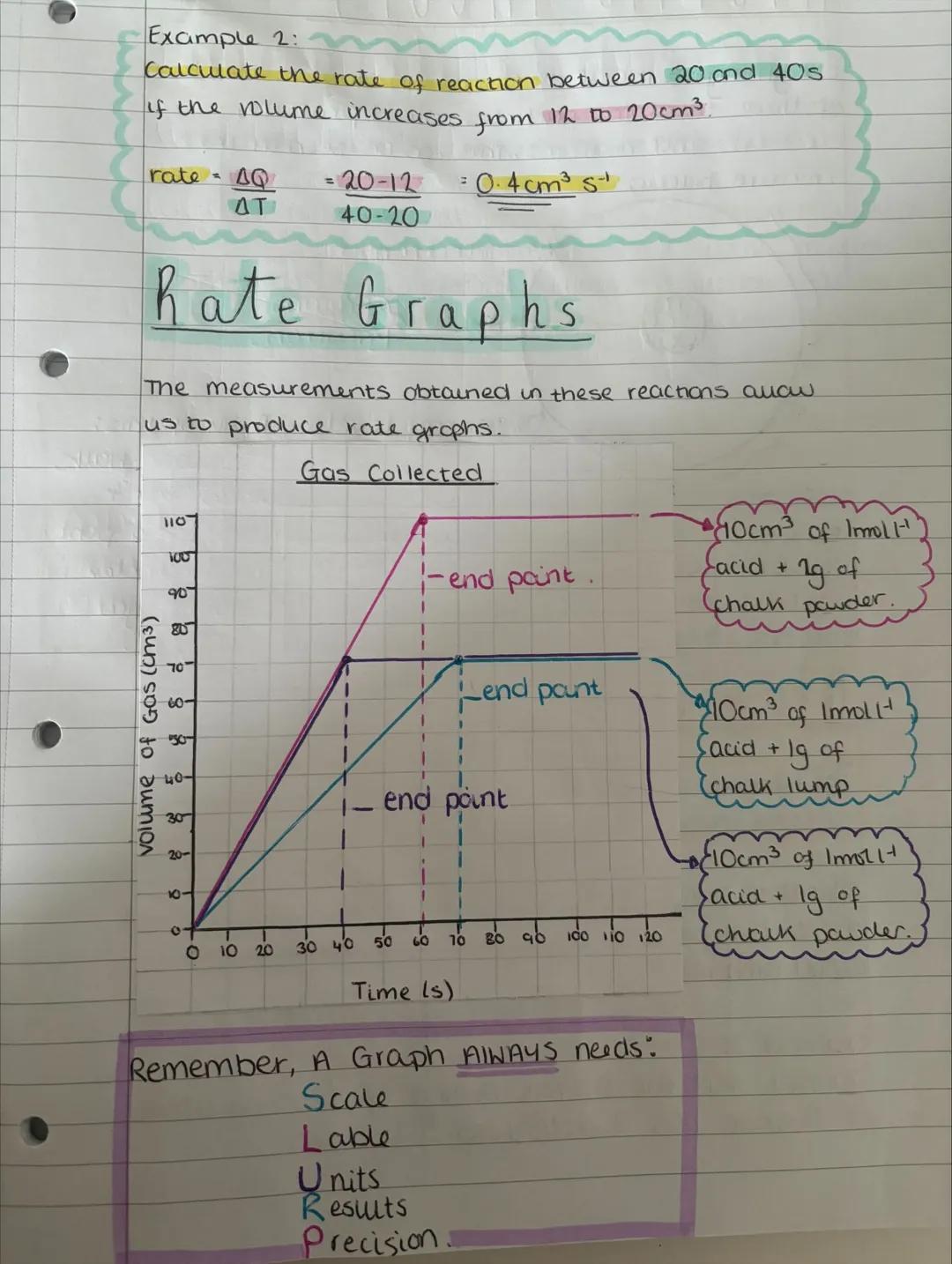
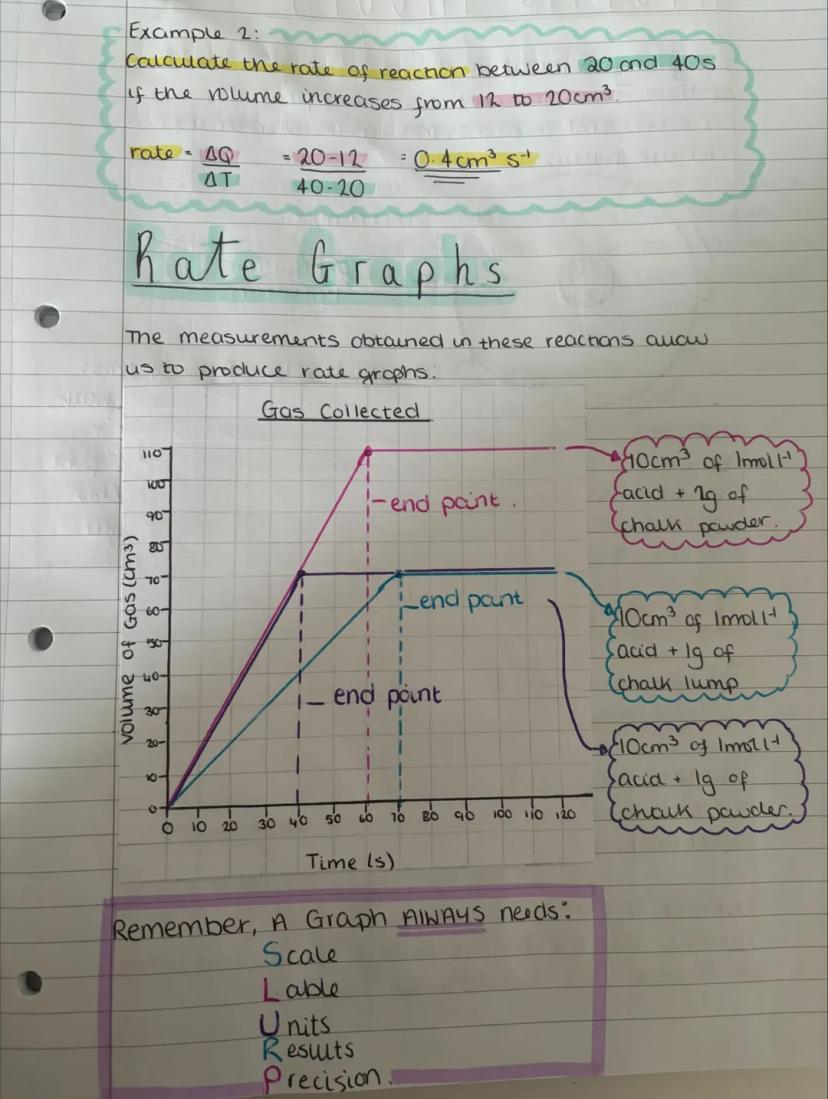
Rate graphs turn your numbers into visual stories about reactions. The steeper the line, the faster the reaction is happening at that moment. Every graph needs proper scales, labels, units, and plotted results - miss any of these and you'll lose marks!
Looking at the example graph, you can see how particle size affects reaction rate. Chalk powder (smaller particles) produces a much steeper initial curve than chalk lumps, proving that surface area really does matter.
The curves eventually flatten out as reactants get used up. This makes perfect sense - fewer reactants available means fewer successful collisions and a slower reaction rate.
Graph Reading: The initial gradient (steepest part) shows the maximum reaction rate when reactant concentrations are highest.
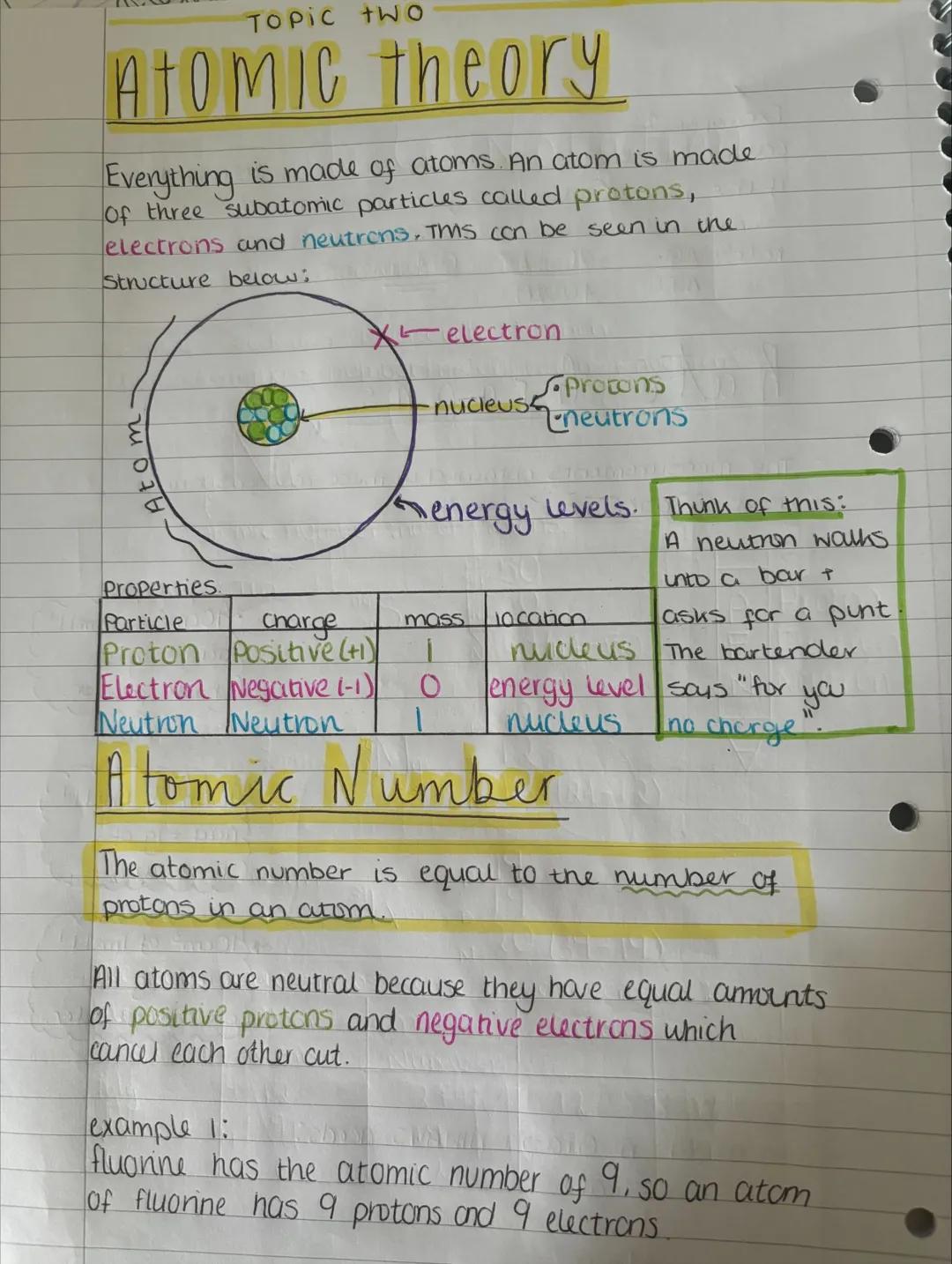
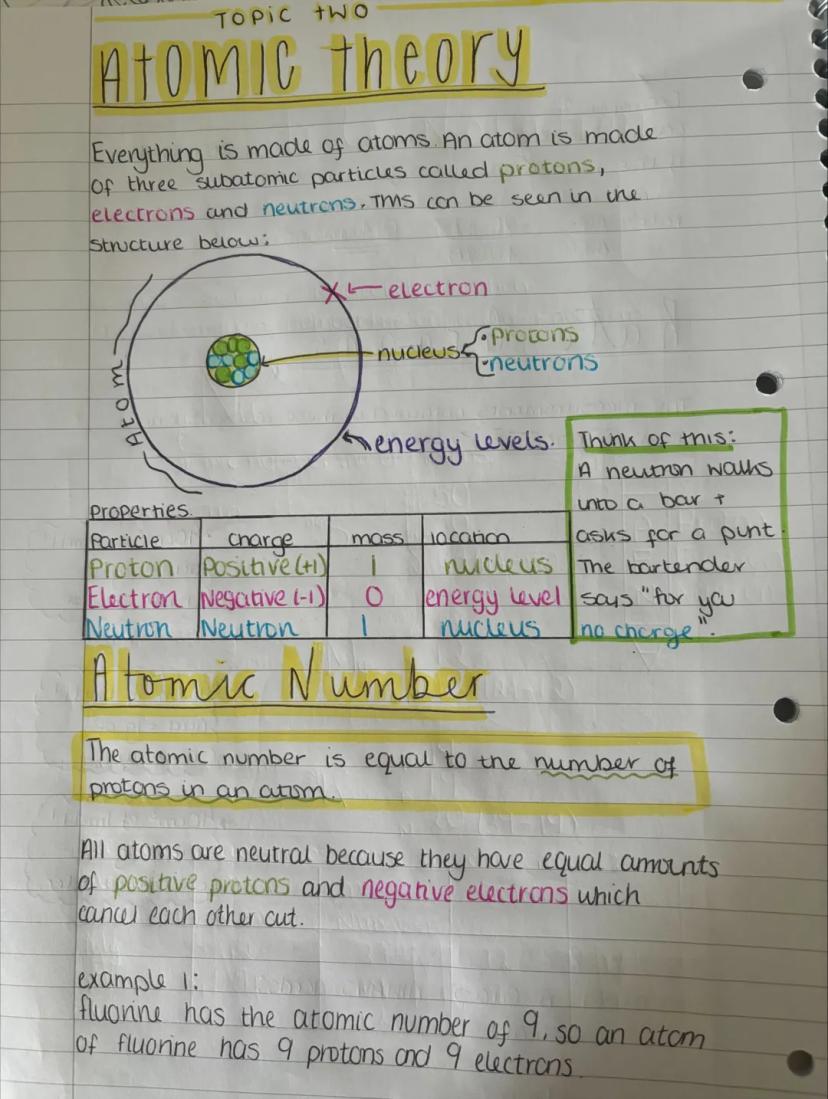
Everything around you is made of atoms - tiny building blocks containing three types of particles. Protons (positive charge) and neutrons (no charge) hang out in the central nucleus, while electrons (negative charge) whiz around in energy levels.
The atomic number tells you how many protons an atom has, and this never changes for a particular element. Atoms are naturally neutral because they have equal numbers of positive protons and negative electrons that cancel each other out.
For example, fluorine has an atomic number of 9, so every fluorine atom has exactly 9 protons and 9 electrons. Change the number of protons and you get a completely different element!
Key Insight: The number of protons defines what element you're dealing with - it's like an atom's unique fingerprint.
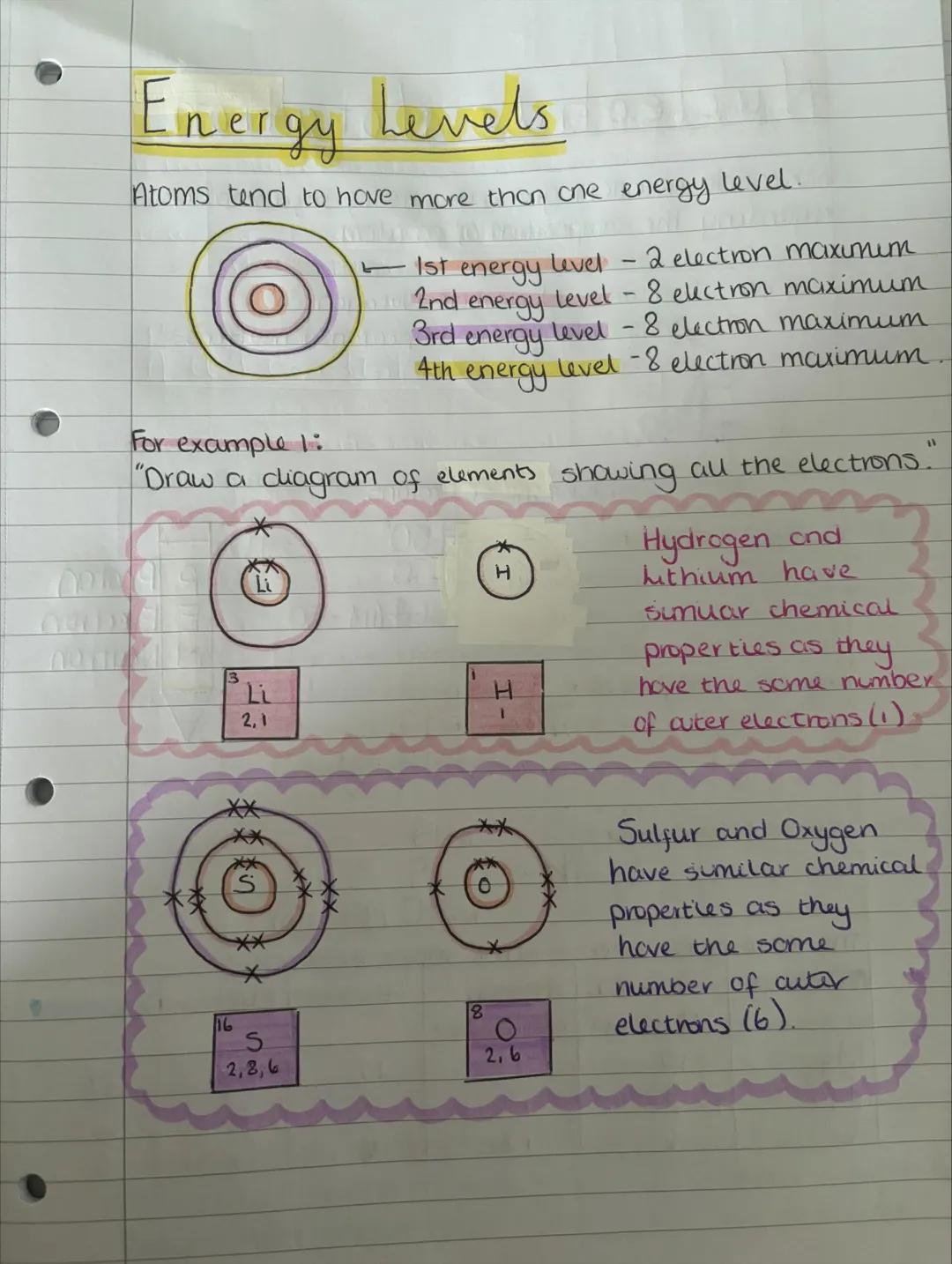
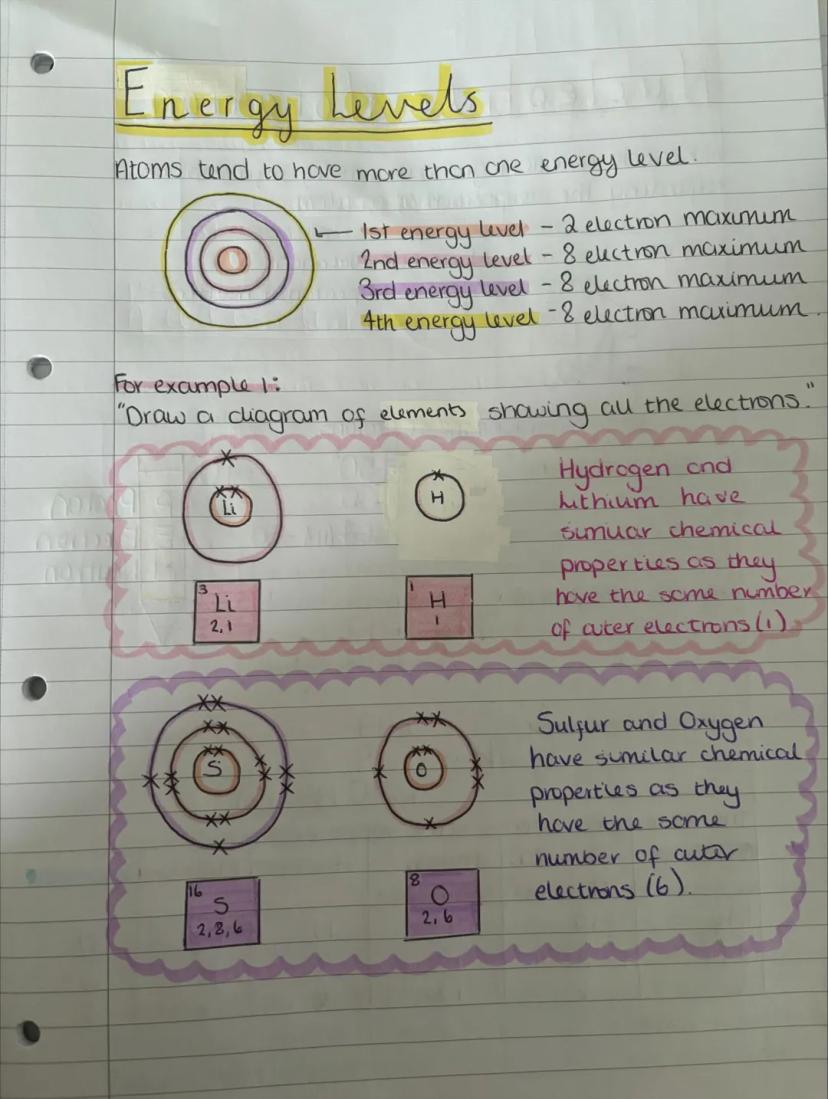
Energy levels around the nucleus can only hold specific numbers of electrons: first level holds 2, and the next three levels each hold 8. Think of them like parking spaces - once they're full, electrons have to use the next level out.
Drawing electron arrangements becomes easy once you know the rules. Hydrogen (1 electron) goes in the first level, while lithium (3 electrons) fills the first level then puts one in the second level: 2,1.
Elements with the same number of outer electrons have similar chemical properties. That's why hydrogen and lithium both react in similar ways - they each have just one electron in their outer level.
Pattern Spotting: Elements in the same group of the periodic table have the same number of outer electrons and similar chemical behaviour.
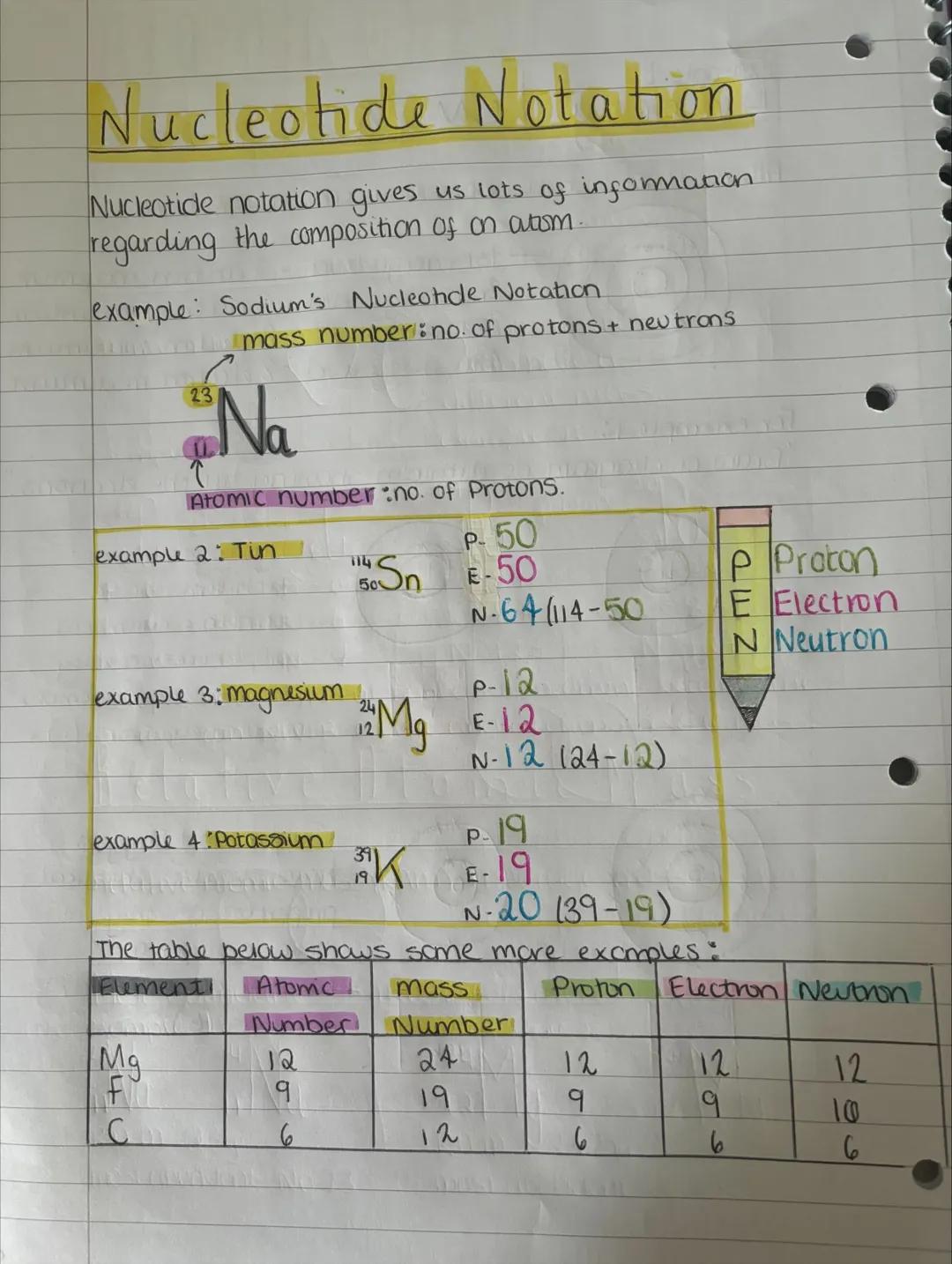
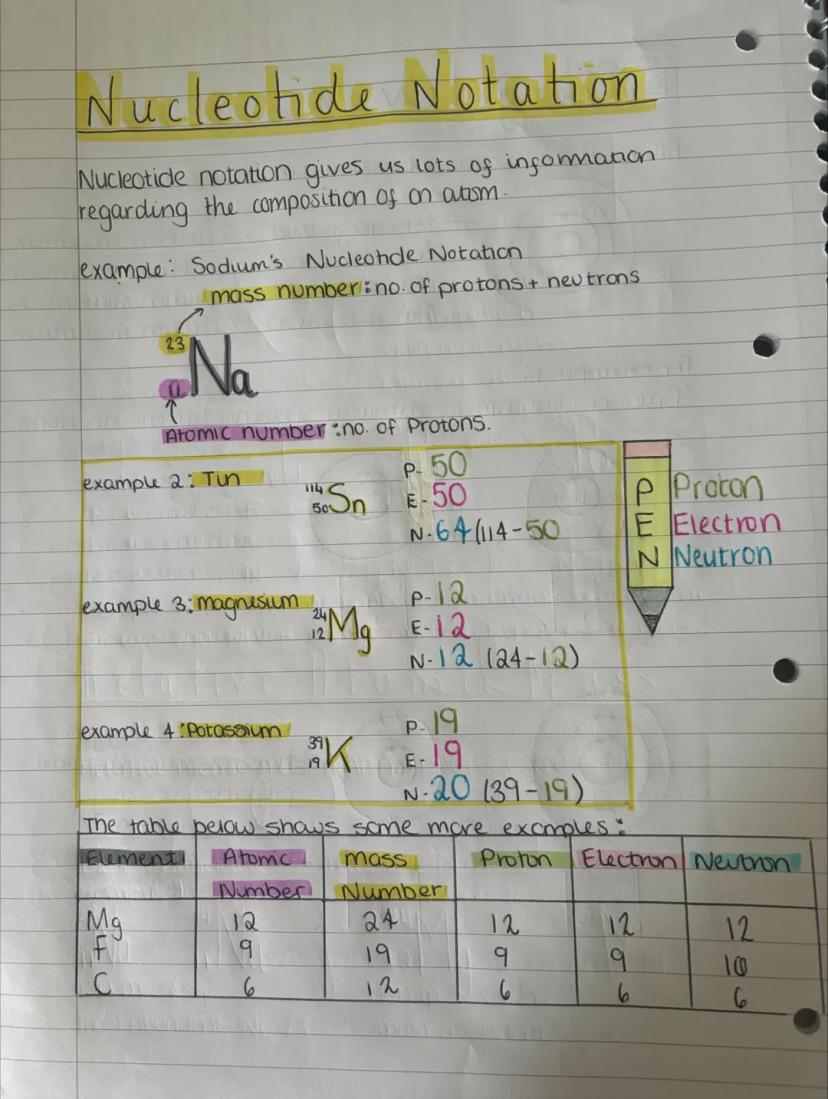
Nuclide notation packs loads of information into a compact format. The big number (mass number) tells you protons plus neutrons, while the small number (atomic number) shows just the protons.
For sodium ²³₁₁Na, you've got 11 protons, 11 electrons, and 12 neutrons (23-11=12). It's like a chemical shorthand that instantly tells you the atom's composition.
Once you know the pattern, you can work out any element's composition. Magnesium ²⁴₁₂Mg has 12 protons, 12 electrons, and 12 neutrons. The maths stays the same every time!
Quick Method: Mass number minus atomic number always gives you the number of neutrons.

Ions are just atoms that have gained or lost electrons, giving them an overall charge. Sodium normally has 11 electrons, but Na⁺ has lost one electron, leaving it with 10 electrons and a positive charge.
Atoms form ions to achieve stable electron arrangements like noble gases. Sodium loses its lone outer electron to get the same arrangement as neon (2,8), while sulfur gains two electrons to match argon's stable 2,8,8 pattern.
It's all about reaching that stable, happy state. Think of it like wanting to be in a complete team rather than being the odd one out - atoms "want" their outer levels to be full.
Memory Hook: Positive ions (like Na⁺) have lost electrons, while negative ions (like S²⁻) have gained electrons.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Amy Neill
@amyneill
Chemistry is everywhere around us - from why food cooks faster when you turn up the heat to how atoms build everything you see. Understanding chemical changes and atomic structure gives you the power to predict and explain what happens... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
You're about to dive into one of the most exciting parts of chemistry! This unit covers everything from reaction rates to atomic structure - basically, how fast things happen and why they happen at all.
Think of it like learning the rules of a game you've been watching your whole life. Once you understand these concepts, you'll start noticing chemistry everywhere - from cooking dinner to understanding why batteries work.
Quick Tip: These topics build on each other, so mastering the basics early will make everything else click into place much easier.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why chips cook faster than whole potatoes? It's all about reaction rates! Four main factors control how fast reactions happen, and once you understand them, you can predict and control chemical reactions.
Temperature is probably the most obvious one. Higher temperatures mean particles move faster, leading to more collisions and quicker reactions. That's why your food lasts ages in the fridge but goes off quickly in a warm room.
Concentration works like a busy corridor - more particles in the same space means more bumping into each other. A 1.0 mol/L acid will react way faster than a 0.1 mol/L acid because there are simply more reactive particles present.
Memory Trick: Think of students moving between classes - more students in a corridor means more collisions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's where things get interesting! Particle size affects reaction rate because smaller particles have a larger surface area. Sugar granules dissolve faster than sugar cubes for exactly this reason - more surface area means more contact with water.
Catalysts are like that friend who stirs up drama but never gets in trouble themselves. They speed up reactions without being used up in the process. A catalyst provides an alternative pathway for the reaction that requires less energy.
The brilliant thing about catalysts is they remain unchanged at the end. Think of a teabag - it helps make your tea but comes out still looking like a teabag!
Real-world Connection: Car catalytic converters use this principle to break down harmful exhaust gases without being consumed themselves.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Now you can actually measure how fast reactions happen! There are several clever ways to collect and measure gases produced during reactions, each suited to different types of gases.
Collecting gas over water only works with gases that won't dissolve in water. You simply let the gas push water out of an upturned measuring cylinder - dead simple but effective.
For soluble gases, you'll need a gas syringe instead. This method captures gases that would otherwise dissolve and disappear into the water. You can also measure mass lost by weighing the reaction flask as gases escape - the mass decreases as products leave the system.
Lab Success: Always check if your gas is soluble in water before choosing your collection method - it'll save you from getting rubbish results!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Displacement methods depend on whether your gas is lighter or heavier than air. Upward displacement works for gases less dense than air, while downward displacement suits gases more dense than air.
Calculating reaction rates uses a straightforward formula: Rate = ΔQ/ΔT. The Greek letter delta (Δ) just means "change in", so you're finding change in quantity over change in time.
For example, if Barry collected 30 cm³ of gas in 20 seconds, his rate would be 30/20 = 1.5 cm³ s⁻¹. Simple maths, but it tells you exactly how fast the reaction is happening!
Exam Tip: Always include units in your rate calculations - typically cm³ s⁻¹ for gas collection experiments.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Rate graphs turn your numbers into visual stories about reactions. The steeper the line, the faster the reaction is happening at that moment. Every graph needs proper scales, labels, units, and plotted results - miss any of these and you'll lose marks!
Looking at the example graph, you can see how particle size affects reaction rate. Chalk powder (smaller particles) produces a much steeper initial curve than chalk lumps, proving that surface area really does matter.
The curves eventually flatten out as reactants get used up. This makes perfect sense - fewer reactants available means fewer successful collisions and a slower reaction rate.
Graph Reading: The initial gradient (steepest part) shows the maximum reaction rate when reactant concentrations are highest.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Everything around you is made of atoms - tiny building blocks containing three types of particles. Protons (positive charge) and neutrons (no charge) hang out in the central nucleus, while electrons (negative charge) whiz around in energy levels.
The atomic number tells you how many protons an atom has, and this never changes for a particular element. Atoms are naturally neutral because they have equal numbers of positive protons and negative electrons that cancel each other out.
For example, fluorine has an atomic number of 9, so every fluorine atom has exactly 9 protons and 9 electrons. Change the number of protons and you get a completely different element!
Key Insight: The number of protons defines what element you're dealing with - it's like an atom's unique fingerprint.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Energy levels around the nucleus can only hold specific numbers of electrons: first level holds 2, and the next three levels each hold 8. Think of them like parking spaces - once they're full, electrons have to use the next level out.
Drawing electron arrangements becomes easy once you know the rules. Hydrogen (1 electron) goes in the first level, while lithium (3 electrons) fills the first level then puts one in the second level: 2,1.
Elements with the same number of outer electrons have similar chemical properties. That's why hydrogen and lithium both react in similar ways - they each have just one electron in their outer level.
Pattern Spotting: Elements in the same group of the periodic table have the same number of outer electrons and similar chemical behaviour.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Nuclide notation packs loads of information into a compact format. The big number (mass number) tells you protons plus neutrons, while the small number (atomic number) shows just the protons.
For sodium ²³₁₁Na, you've got 11 protons, 11 electrons, and 12 neutrons (23-11=12). It's like a chemical shorthand that instantly tells you the atom's composition.
Once you know the pattern, you can work out any element's composition. Magnesium ²⁴₁₂Mg has 12 protons, 12 electrons, and 12 neutrons. The maths stays the same every time!
Quick Method: Mass number minus atomic number always gives you the number of neutrons.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ions are just atoms that have gained or lost electrons, giving them an overall charge. Sodium normally has 11 electrons, but Na⁺ has lost one electron, leaving it with 10 electrons and a positive charge.
Atoms form ions to achieve stable electron arrangements like noble gases. Sodium loses its lone outer electron to get the same arrangement as neon (2,8), while sulfur gains two electrons to match argon's stable 2,8,8 pattern.
It's all about reaching that stable, happy state. Think of it like wanting to be in a complete team rather than being the odd one out - atoms "want" their outer levels to be full.
Memory Hook: Positive ions (like Na⁺) have lost electrons, while negative ions (like S²⁻) have gained electrons.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
23
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the essential techniques and calculations involved in acid-base titrations. This summary covers the titration process, including the use of burettes, pipettes, indicators, and how to determine the concentration of unknown acids or alkalis through neutralization reactions. Ideal for chemistry students preparing for exams or practical assessments.
Explore key concepts in acids, bases, and buffers through detailed practice questions and model answers. This resource covers acid-base titrations, pKa values, conjugate acid-base pairs, and the Henderson-Hasselbalch equation, providing essential calculations and explanations for mastering the topic.
Explore the fundamentals of buffer solutions, including the role of weak acids and bases, pH calculations, and the Henderson-Hasselbalch equation. This summary covers key concepts such as buffer capacity and partial neutralization, providing essential calculations for A-Level Chemistry students.
Explore the fundamentals of Brønsted-Lowry acid-base theory, pH calculations, and buffer solutions. This comprehensive guide covers key concepts such as acid-base equilibria, the ionic product of water (Kw), dissociation constants (Ka, pKa), and practical applications in acid-base titrations. Ideal for AQA A-Level Physical Chemistry students.
Explore a comprehensive set of exam questions focused on weak acids and bases, including topics such as acid-base titrations, buffer solutions, and the acid dissociation constant (Ka). This resource is ideal for students preparing for chemistry exams, providing practice on key concepts like pH calculations, neutralization reactions, and the behavior of carboxylic acids.
Explore the essential techniques of acid-base titrations, including key definitions, step-by-step methods, and calculations for determining unknown concentrations and molar masses. This summary also covers measurement uncertainties and how to effectively record results. Ideal for chemistry students preparing for practical assessments.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user