Introduction to Organic Chemistry
Ever wondered how chemists make sense of millions of different carbon compounds? It all starts with understanding the different ways to write chemical formulas.
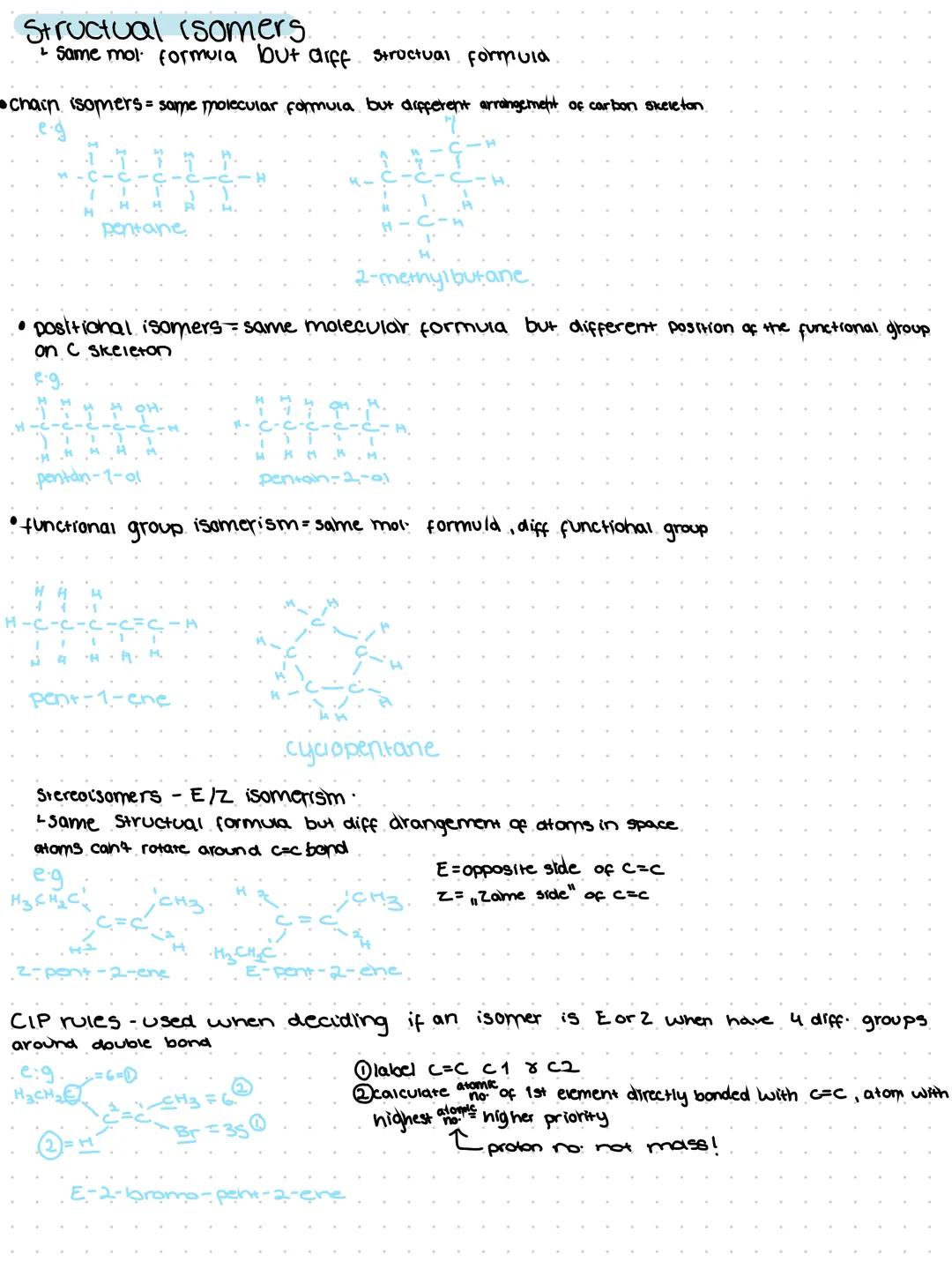
General formulas give you the pattern for entire families of compounds (like CₙH₂ₙ₊₂ for alkanes). Empirical formulas show the simplest ratio of atoms, whilst structural formulas reveal how atoms connect without showing every bond. Skeletal formulas are the shorthand version - just carbon bonds with functional groups visible.
Homologous series are families of compounds sharing the same functional group and general formula. Think alkanes −ane, alkenes −ene, alcohols −ol, and carboxylic acids −oicacid. Each series follows predictable patterns that make learning much easier.
The IUPAC naming system is chemistry's universal language. Find the longest carbon chain, identify the functional group for your suffix, number the chain so the functional group gets the lowest possible number, then add any side chains as prefixes in alphabetical order.
Quick Tip: Always start curly arrows from areas with electron pairs (like double bonds or lone pairs) - they show exactly how electrons move during reactions.