Higher Chemistry covers the essential topics you'll need to master... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
275
•
5 Feb 2026
•
fasai✧
@myfxsai
Higher Chemistry covers the essential topics you'll need to master... Show more




Higher Chemistry is divided into four main areas that build on each other brilliantly. Chemical Changes and Structure forms your foundation, covering how elements are organised and why they behave differently.
Nature's Chemistry gets into the fascinating world of organic compounds - think alcohols, proteins, and even the chemistry behind your skincare products. Meanwhile, Chemistry in Society shows you how chemical principles solve real-world problems, from maximising yields to analysing unknown substances.
Finally, Researching Chemistry gives you the practical skills you'll need for coursework and lab work. Each section connects to the others, so mastering the basics early on will make the advanced topics much easier to understand.
Quick Tip: Don't try to memorise everything at once - focus on understanding the patterns and relationships between topics instead.
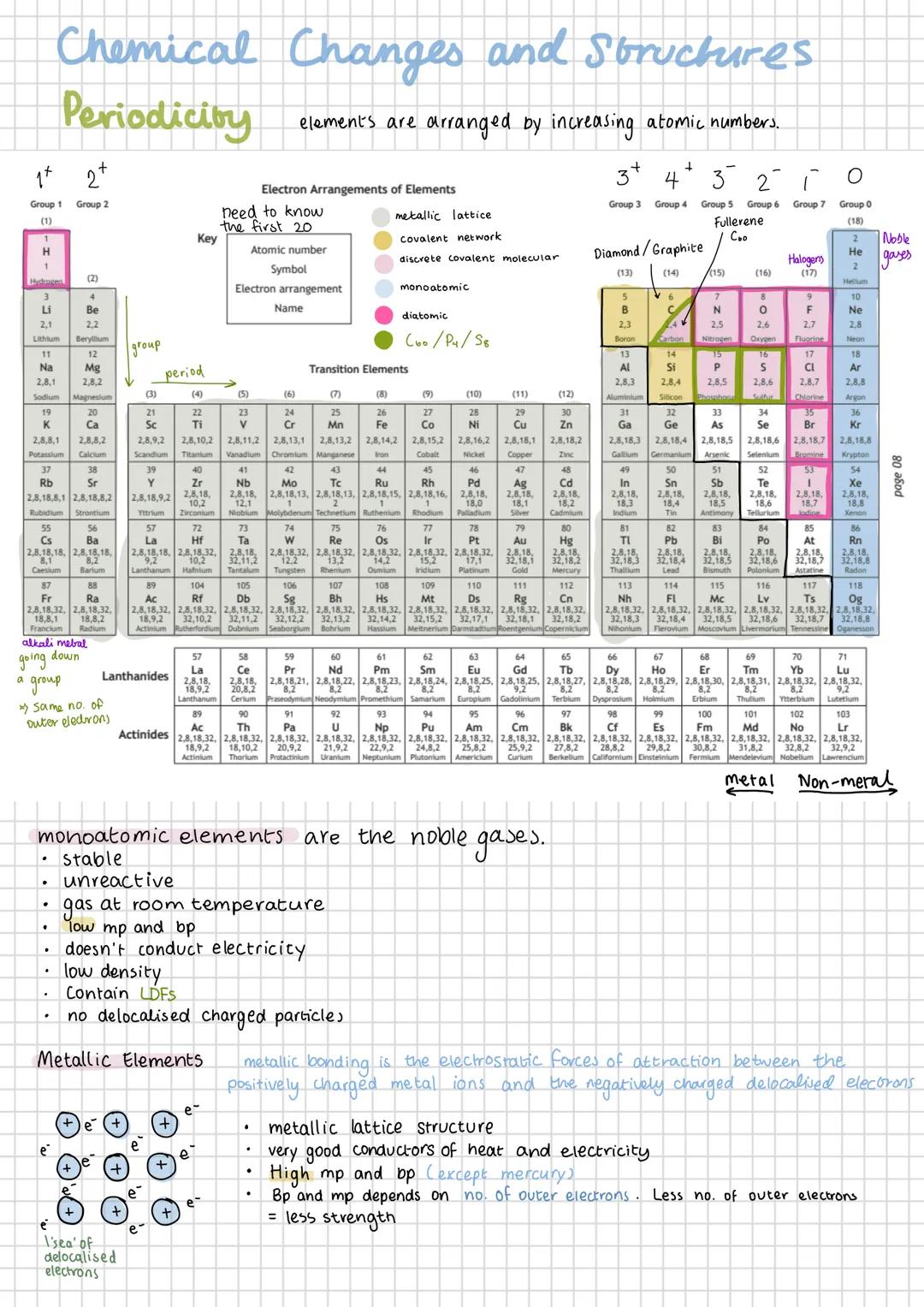
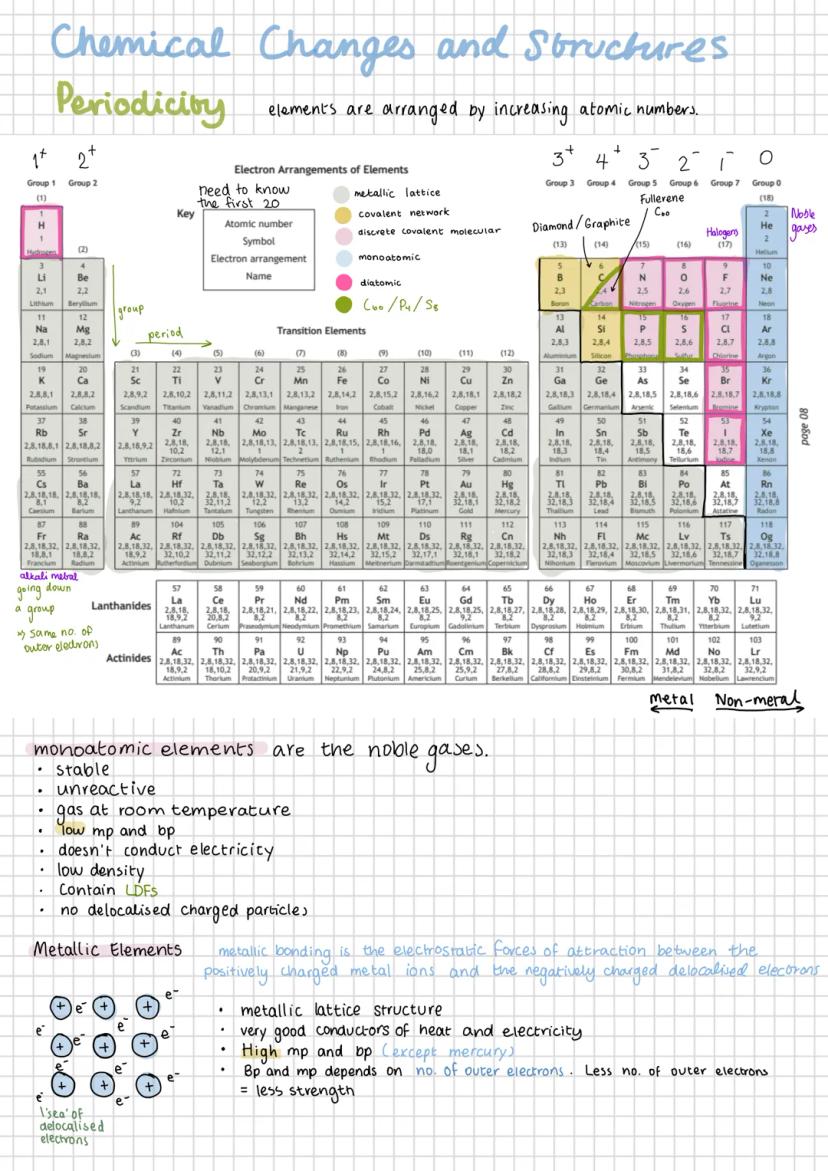
The periodic table isn't just a random arrangement of elements - it's organised by increasing atomic number, which creates predictable patterns you can use to your advantage. Elements in the same group have the same number of outer electrons, whilst those in the same period have the same number of electron shells.
You absolutely must know the electron arrangements of the first 20 elements - they're the key to understanding chemical behaviour. For example, sodium (2,8,1) and potassium (2,8,8,1) both have one outer electron, which explains why they react similarly.
Elements can be classified by their structure: metallic lattice (like iron), covalent network (like diamond), discrete covalent molecular (like oxygen), or monoatomic (like helium). Each structure type has characteristic properties that make perfect sense once you understand the bonding involved.
The noble gases are particularly important because they're stable and unreactive. They exist as single atoms with complete outer shells, have low melting and boiling points, and don't conduct electricity because they lack delocalised electrons.
Remember: Group number = number of outer electrons, Period number = number of electron shells. This simple rule unlocks so much chemistry!
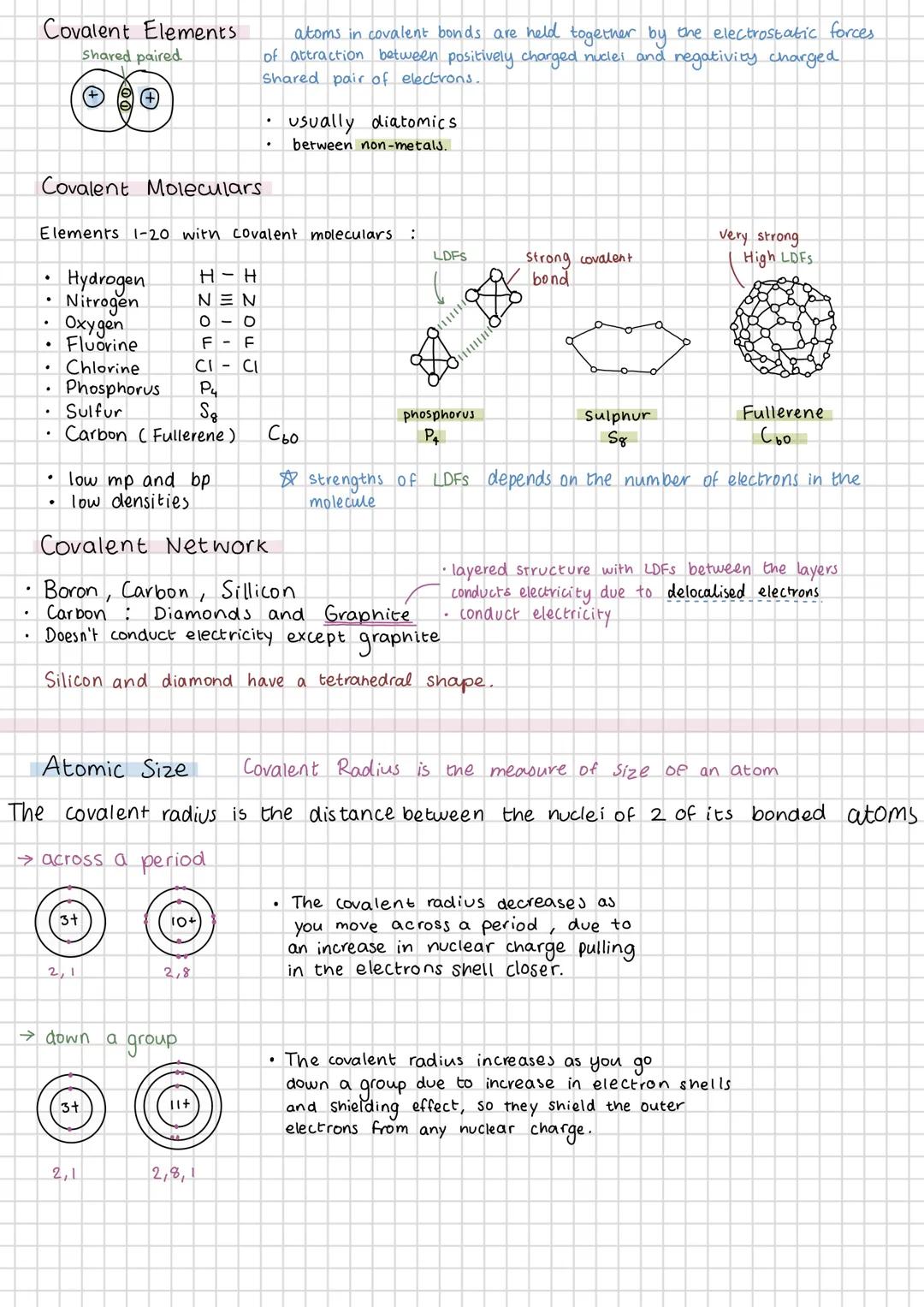
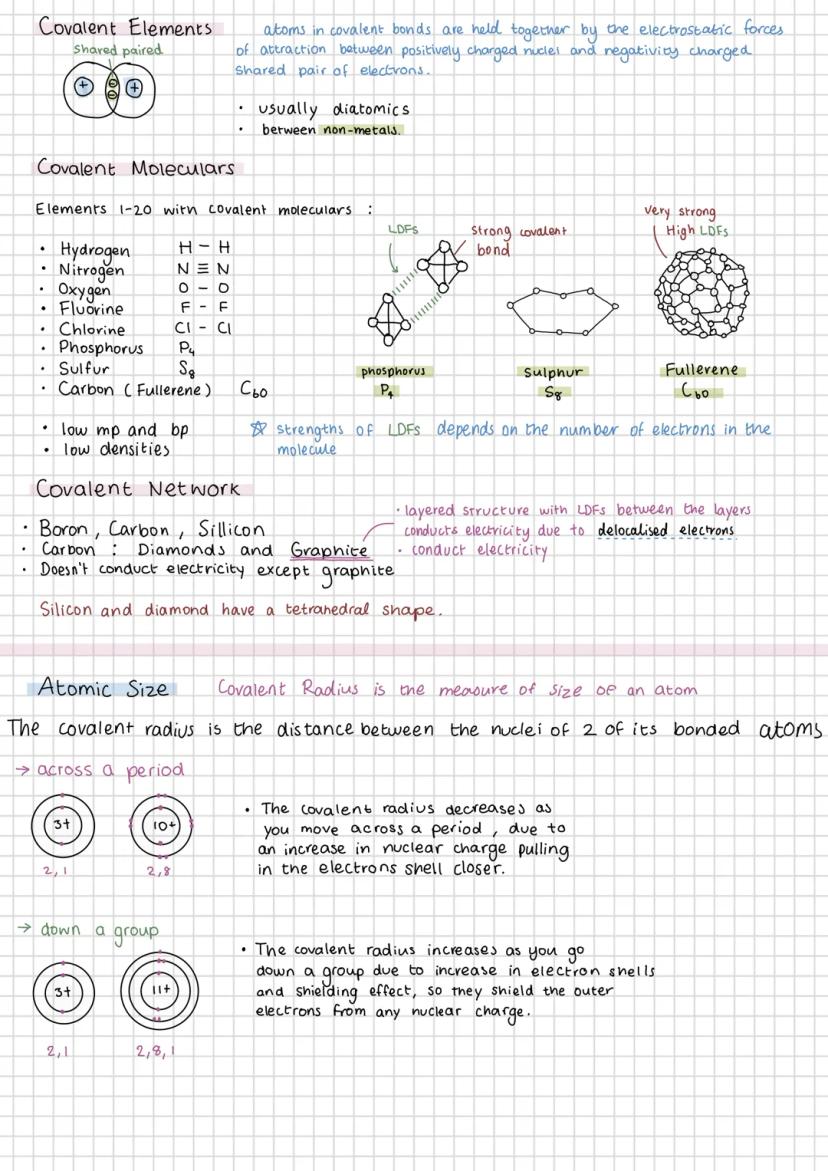
Covalent bonding occurs when atoms share electrons, typically between non-metals. You'll encounter covalent molecular elements like H₂, N₂, O₂, and larger molecules like P₄ and S₈. These have low melting points because the intermolecular forces are weak, even though the covalent bonds within molecules are strong.
Covalent network structures like diamond and graphite are completely different beasts. They have extremely high melting points because breaking them means breaking covalent bonds throughout the structure. Graphite's unique because it conducts electricity due to delocalised electrons between its layers.
Covalent radius decreases across a period because increasing nuclear charge pulls electrons closer. Going down a group, it increases because you're adding electron shells, and the shielding effect reduces the pull on outer electrons.
Understanding these trends helps you predict properties. Silicon and diamond both have tetrahedral structures, whilst graphite's layered structure with weak forces between layers explains why you can write with pencil lead.
Visual Aid: Picture the nucleus as a magnet - more protons mean stronger pull on electrons, but more electron shells create a 'shielding' effect.
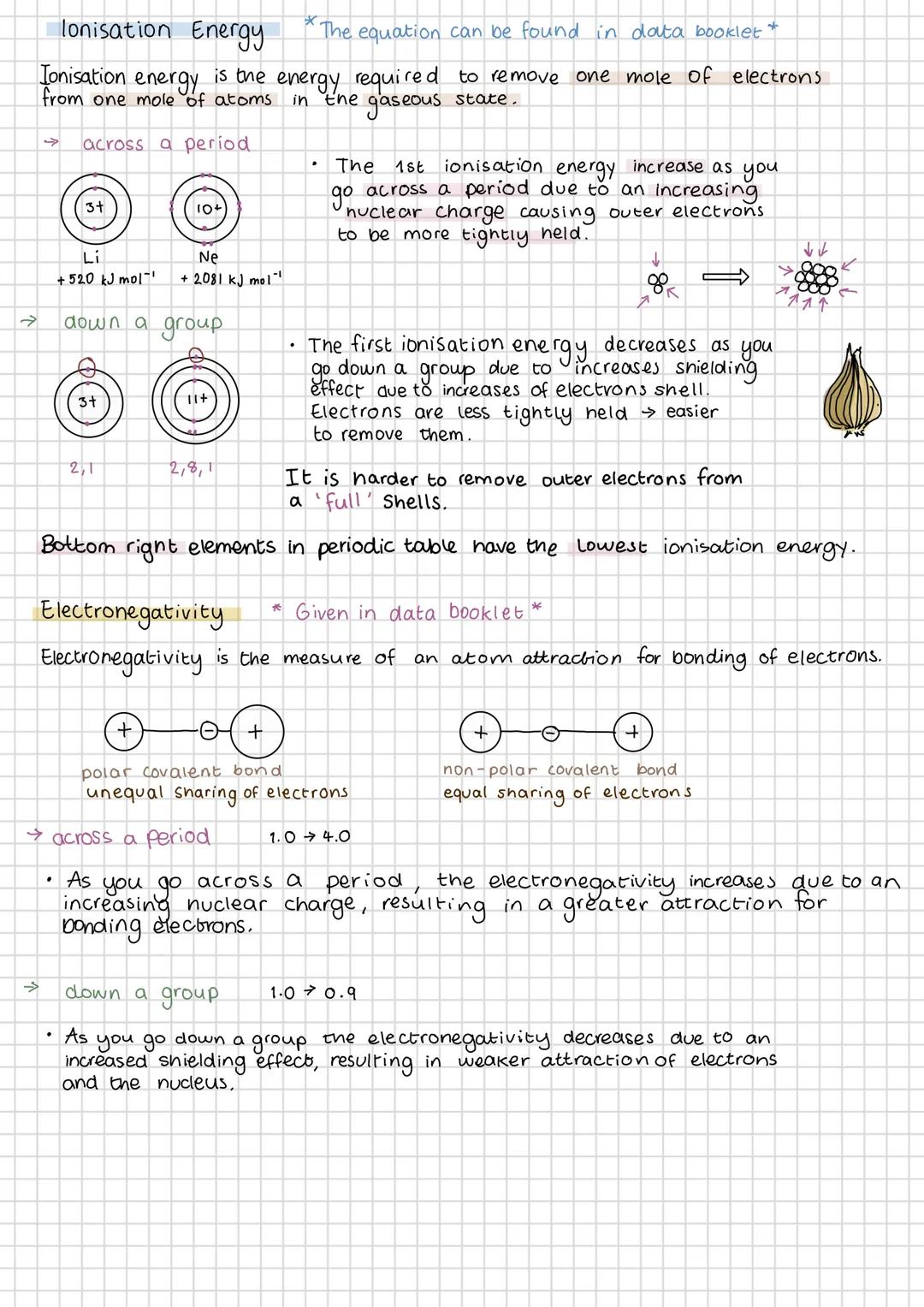
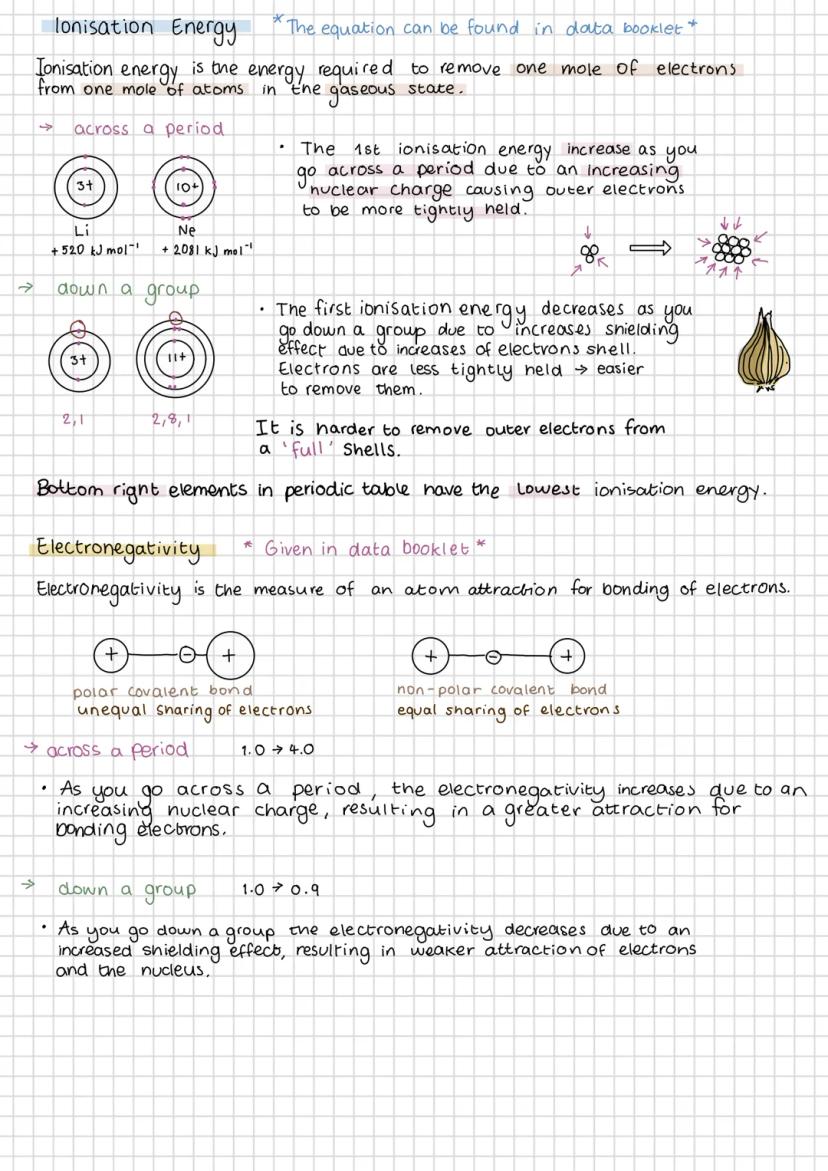
Ionisation energy is the energy needed to remove an electron from an atom in the gas phase. It increases across a period because stronger nuclear charge holds electrons more tightly, but decreases down a group due to increased shielding from extra electron shells.
Elements in the bottom left of the periodic table have the lowest ionisation energies - they lose electrons most easily. This explains why Group 1 metals are so reactive and why they form positive ions readily.
Electronegativity measures how strongly an atom attracts bonding electrons. It follows the same trends as ionisation energy - increasing across periods and decreasing down groups. The values are given in your data booklet, so you don't need to memorise them.
The difference in electronegativity between bonded atoms determines bond type. Small differences (0.0-0.4) give non-polar covalent bonds with equal electron sharing. Larger differences create polar covalent bonds where electrons are pulled towards the more electronegative atom, creating partial charges .
Exam Tip: Always check if molecular shapes are symmetrical - even polar bonds can cancel out to give non-polar molecules overall!

Non-polar covalent bonding occurs when atoms have identical or very similar electronegativity values . Think F₂ or C-H bonds - electrons are shared equally between atoms, creating no partial charges.
Polar covalent bonding happens with moderate electronegativity differences. The more electronegative atom gets a partial negative charge (δ-) whilst the other becomes partially positive (δ+). Water's O-H bonds are perfect examples - oxygen pulls electrons away from hydrogen.
Here's the crucial bit: even molecules with polar bonds can be non-polar overall if they're symmetrical. CO₂ has polar C=O bonds, but because it's linear, the polarities cancel out. Always consider molecular shape when predicting polarity.
Ionic bonding forms when electronegativity differences are large (usually >1.7). Electrons transfer completely from metal to non-metal, creating charged ions held together by electrostatic attraction. Remember that metals typically form positive ions, non-metals form negative ones.
Memory Trick: Think of electronegativity like a tug-of-war - small difference means equal pull, big difference means one side wins completely!
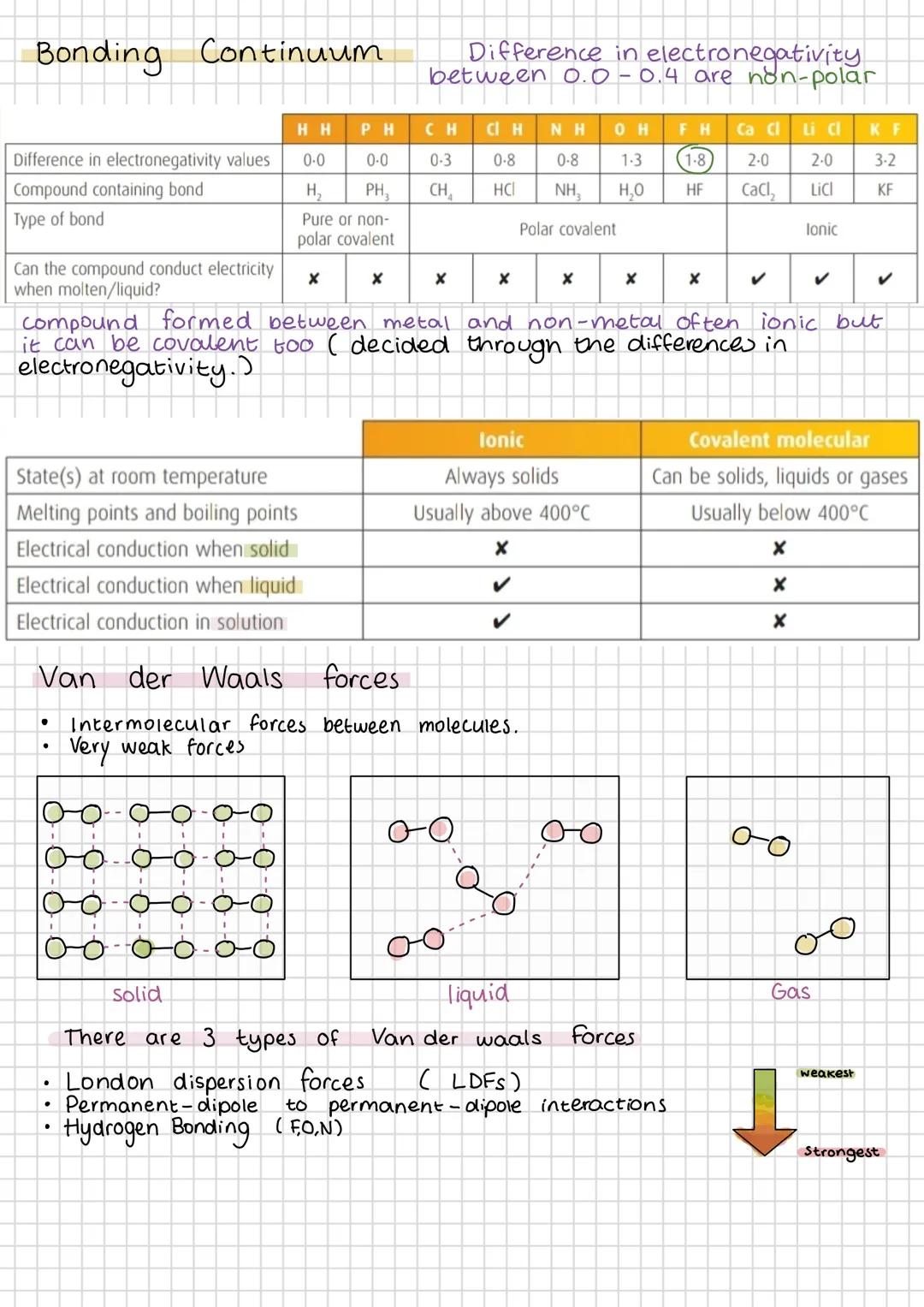
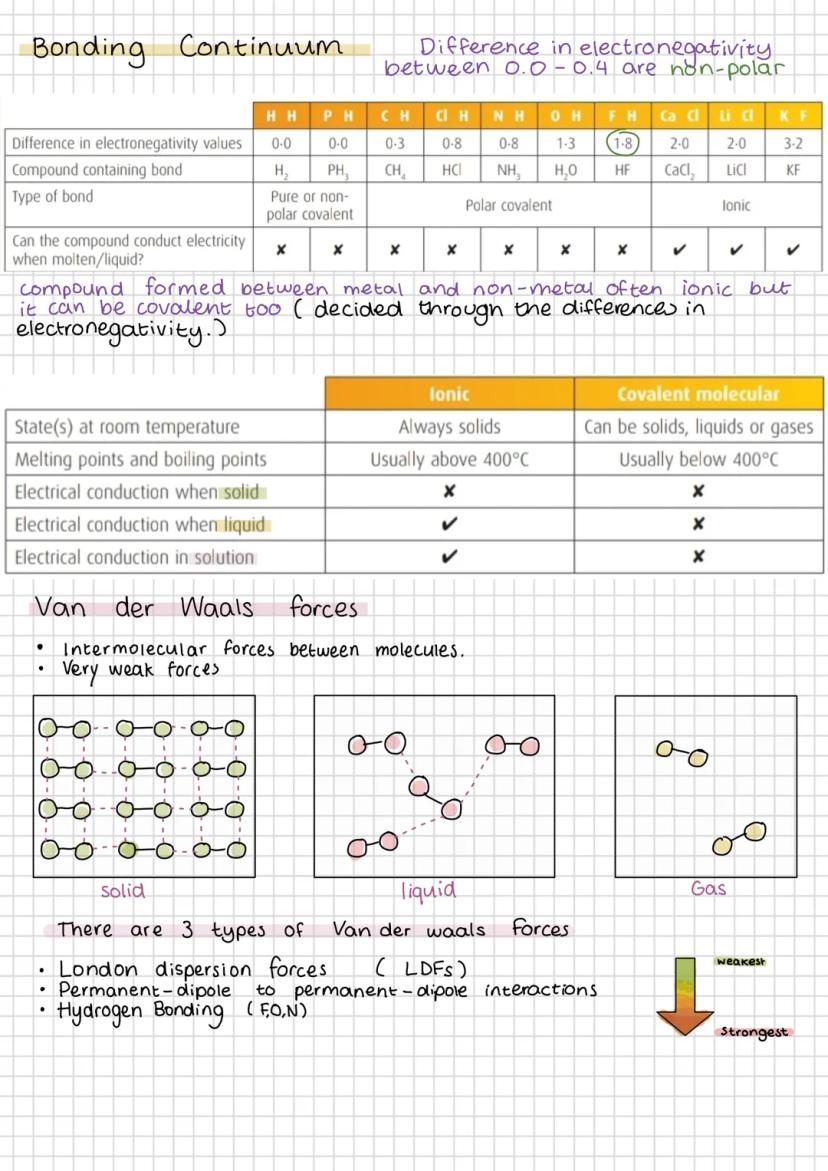
The bonding continuum shows how bond types blend into each other based on electronegativity differences. Pure covalent (0.0-0.4) gradually becomes polar covalent, then ionic as differences increase. Don't expect sharp boundaries - chemistry loves gradual transitions.
Ionic compounds always conduct electricity when molten or dissolved because ions can move freely. Covalent compounds never conduct in any state because they don't have mobile charged particles. This difference is crucial for identifying compound types.
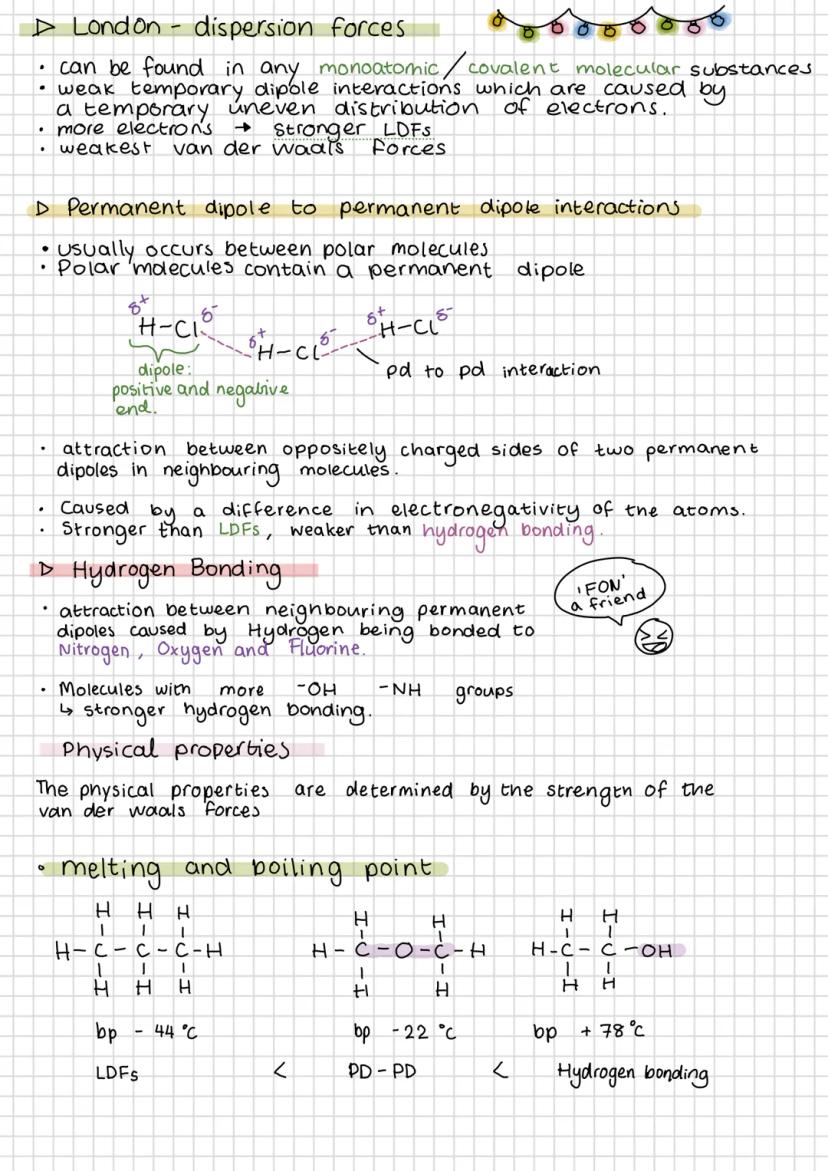
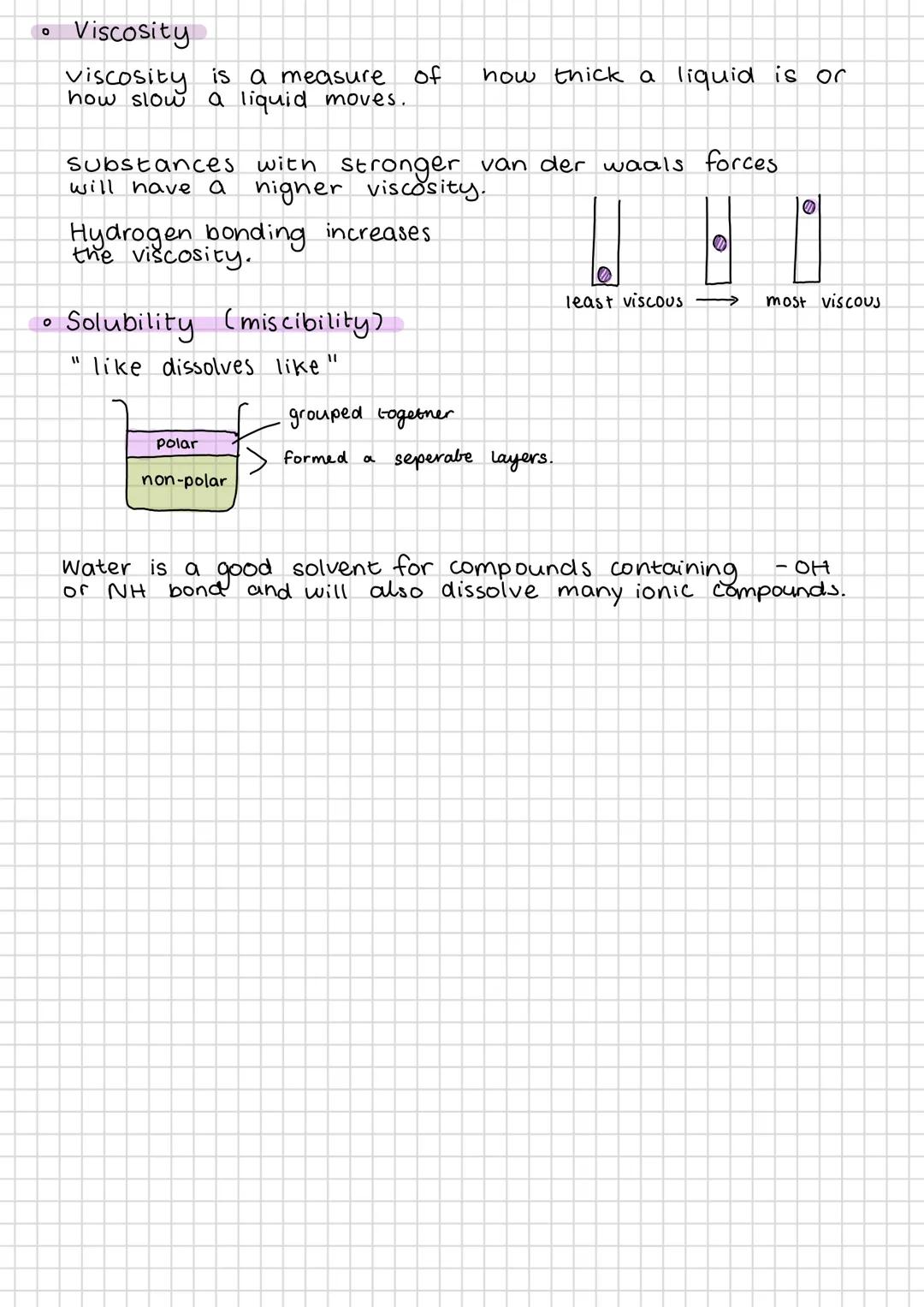
Van der Waals forces are weak intermolecular attractions that exist between all molecules. London dispersion forces (LDFs) are the weakest and occur everywhere due to temporary electron movement. More electrons mean stronger LDFs.
Permanent dipole-dipole interactions occur between polar molecules - the δ+ end of one molecule attracts the δ- end of another. Hydrogen bonding is the strongest intermolecular force, occurring when hydrogen bonds to F, O, or N (remember "FON").
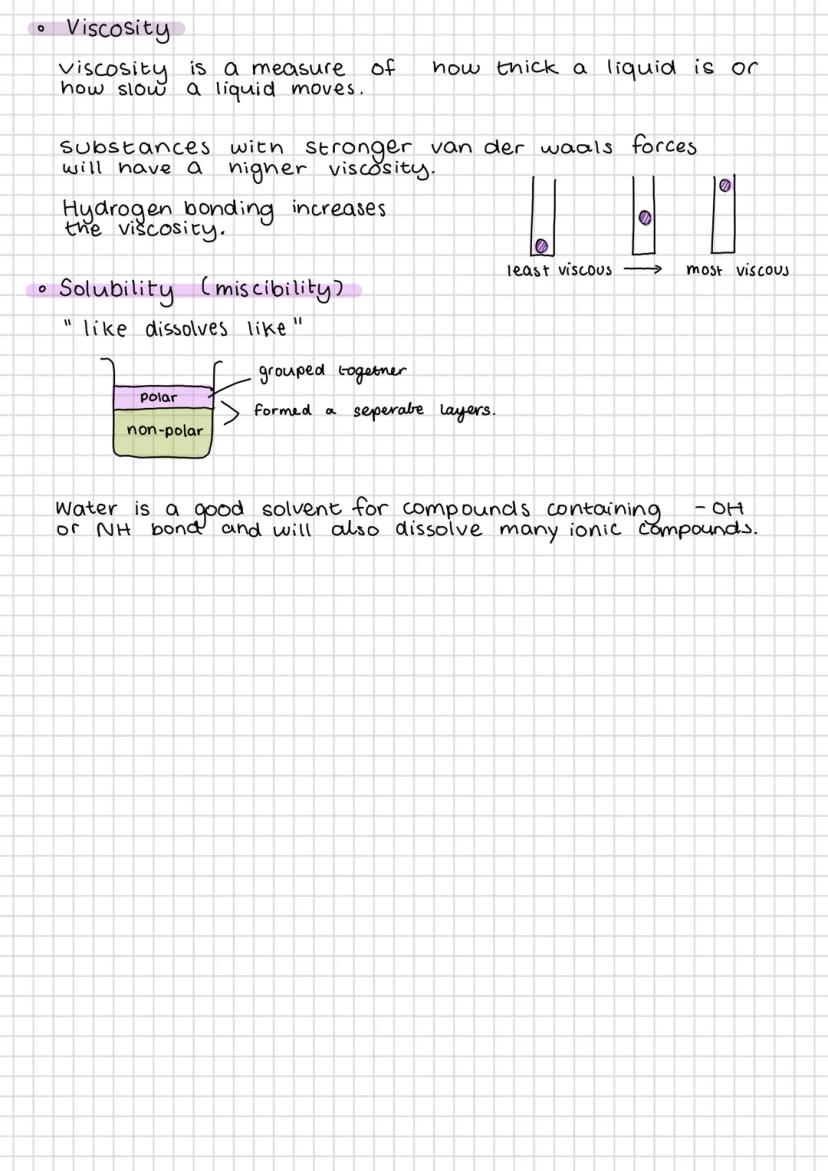
Key Point: Intermolecular forces determine physical properties like boiling points - stronger forces mean higher temperatures needed to separate molecules.
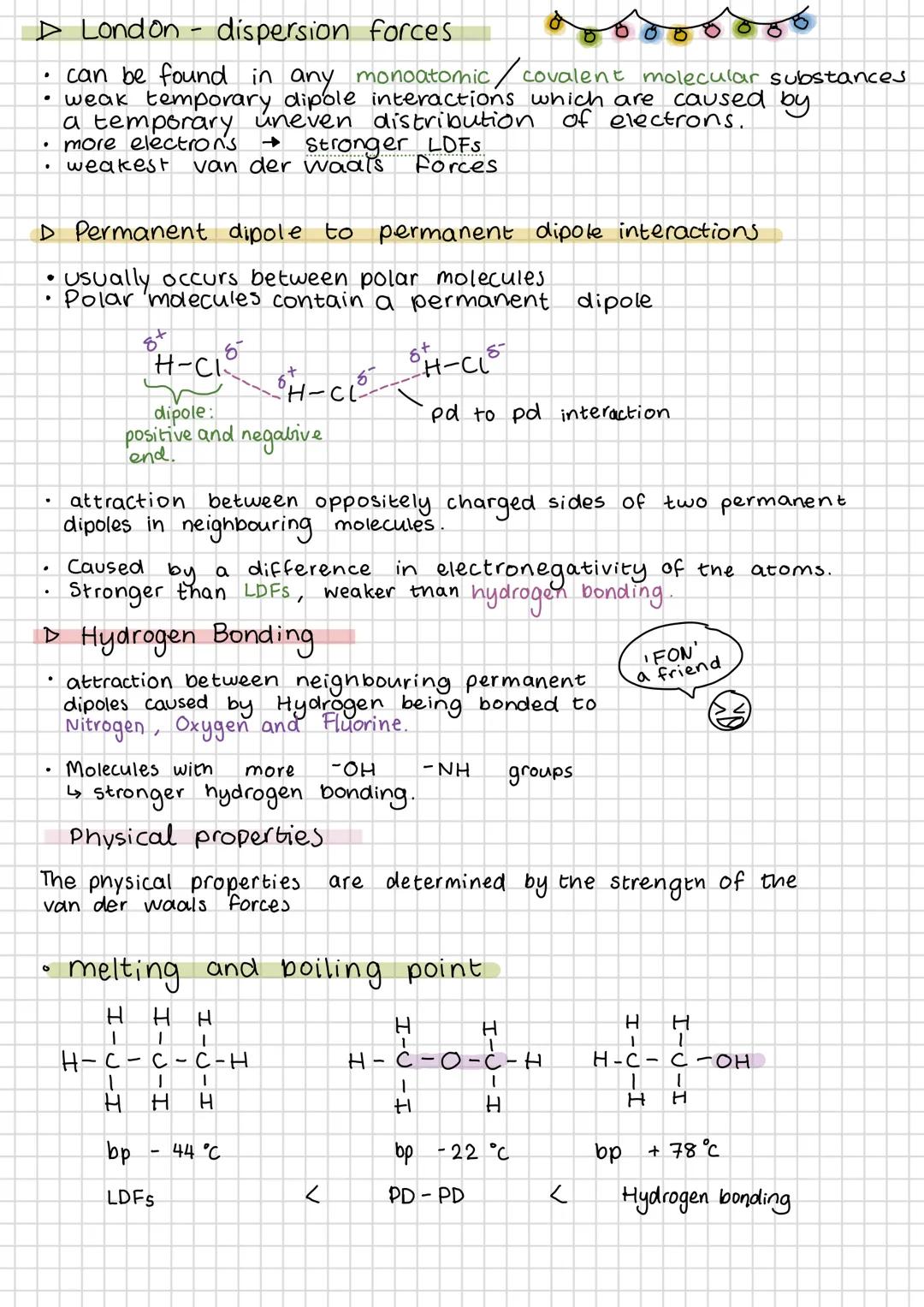
London dispersion forces exist in all substances and get stronger with more electrons. They're caused by temporary, uneven electron distribution creating temporary dipoles that attract neighbouring molecules.
Permanent dipole-dipole interactions occur between polar molecules where the positive end of one molecule attracts the negative end of another. These are stronger than LDFs but weaker than hydrogen bonds.
Hydrogen bonding only happens when hydrogen is directly bonded to fluorine, oxygen, or nitrogen. The small size and high electronegativity of these atoms create particularly strong dipoles. More -OH or -NH groups mean stronger hydrogen bonding overall.
The strength of intermolecular forces directly determines melting and boiling points. Propane (only LDFs) boils at -44°C, dimethyl ether at -22°C, but ethanol (hydrogen bonding) at +78°C. This pattern shows you can predict properties from molecular structure.
Practical Application: Understanding these forces explains why alcohol has a higher boiling point than expected for its size - it's all about those hydrogen bonds!
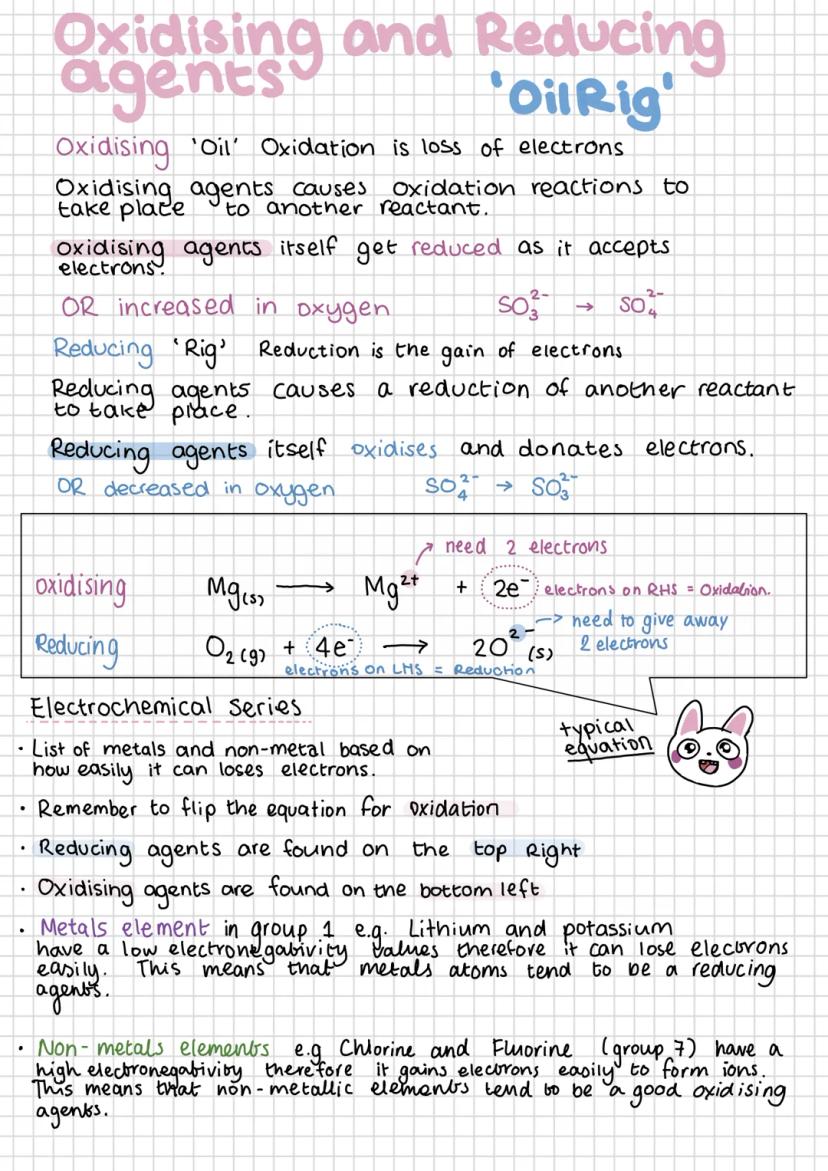
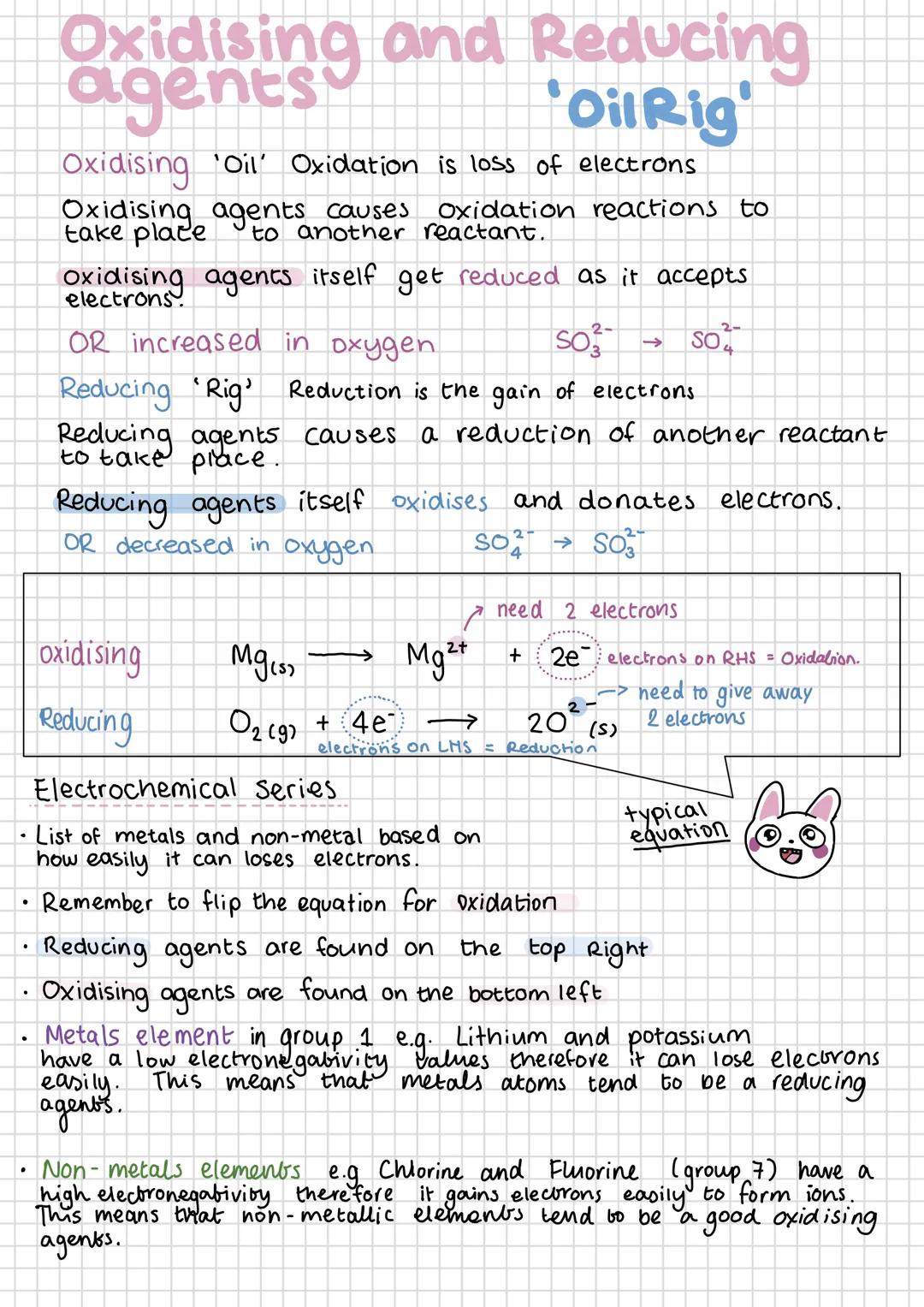
Remember "OIL RIG" - Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons). Oxidising agents cause other substances to be oxidised whilst getting reduced themselves. Reducing agents do the opposite - they cause reduction whilst being oxidised.
The electrochemical series ranks elements by how easily they lose electrons. Strong reducing agents (like lithium and potassium) appear at the top - they lose electrons readily due to low electronegativity. Strong oxidising agents (like fluorine and chlorine) appear at the bottom.
Group 1 metals make excellent reducing agents because they have low electronegativity and lose their outer electron easily. Group 7 elements are powerful oxidising agents because they readily gain electrons to complete their outer shells.
Common examples include hydrogen peroxide (H₂O₂) as a strong oxidising agent used in cleaning products and bleach, and carbon monoxide (CO) as a reducing agent. Oxidising agents kill bacteria and break down coloured compounds, explaining their use in disinfectants and bleaches.
Exam Strategy: When balancing redox equations, always balance elements first, then add H₂O for oxygen, H⁺ for hydrogen, and finally electrons for charge.
Hydrogen peroxide (H₂O₂) is a powerful oxidising agent you'll encounter frequently. It's used in cleaning products because it kills fungi and bacteria, and in bleaches because it breaks down coloured compounds by oxidising them.
Carbon monoxide (CO) serves as an important reducing agent, particularly in industrial processes like metal extraction where it removes oxygen from metal oxides.
Redox equations must balance both mass and charge. Start by balancing the main elements, then add H₂O molecules to balance oxygen atoms, H⁺ ions to balance hydrogen atoms, and finally electrons to balance the overall charge.
Displacement reactions follow predictable patterns using the electrochemical series. A more reactive metal (higher up the series) will displace a less reactive metal from its compounds. For example, zinc displaces silver from silver nitrate because zinc is higher in the series and more easily oxidised.
Real-world Connection: Understanding redox reactions explains everything from how batteries work to why iron rusts - it's oxidation and reduction happening all around us!

Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
fasai✧
@myfxsai
Higher Chemistry covers the essential topics you'll need to master for your A-levels, from understanding how atoms behave in the periodic table to exploring the chemistry behind everyday products. This comprehensive guide breaks down complex concepts into digestible sections that'll... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Higher Chemistry is divided into four main areas that build on each other brilliantly. Chemical Changes and Structure forms your foundation, covering how elements are organised and why they behave differently.
Nature's Chemistry gets into the fascinating world of organic compounds - think alcohols, proteins, and even the chemistry behind your skincare products. Meanwhile, Chemistry in Society shows you how chemical principles solve real-world problems, from maximising yields to analysing unknown substances.
Finally, Researching Chemistry gives you the practical skills you'll need for coursework and lab work. Each section connects to the others, so mastering the basics early on will make the advanced topics much easier to understand.
Quick Tip: Don't try to memorise everything at once - focus on understanding the patterns and relationships between topics instead.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The periodic table isn't just a random arrangement of elements - it's organised by increasing atomic number, which creates predictable patterns you can use to your advantage. Elements in the same group have the same number of outer electrons, whilst those in the same period have the same number of electron shells.
You absolutely must know the electron arrangements of the first 20 elements - they're the key to understanding chemical behaviour. For example, sodium (2,8,1) and potassium (2,8,8,1) both have one outer electron, which explains why they react similarly.
Elements can be classified by their structure: metallic lattice (like iron), covalent network (like diamond), discrete covalent molecular (like oxygen), or monoatomic (like helium). Each structure type has characteristic properties that make perfect sense once you understand the bonding involved.
The noble gases are particularly important because they're stable and unreactive. They exist as single atoms with complete outer shells, have low melting and boiling points, and don't conduct electricity because they lack delocalised electrons.
Remember: Group number = number of outer electrons, Period number = number of electron shells. This simple rule unlocks so much chemistry!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Covalent bonding occurs when atoms share electrons, typically between non-metals. You'll encounter covalent molecular elements like H₂, N₂, O₂, and larger molecules like P₄ and S₈. These have low melting points because the intermolecular forces are weak, even though the covalent bonds within molecules are strong.
Covalent network structures like diamond and graphite are completely different beasts. They have extremely high melting points because breaking them means breaking covalent bonds throughout the structure. Graphite's unique because it conducts electricity due to delocalised electrons between its layers.
Covalent radius decreases across a period because increasing nuclear charge pulls electrons closer. Going down a group, it increases because you're adding electron shells, and the shielding effect reduces the pull on outer electrons.
Understanding these trends helps you predict properties. Silicon and diamond both have tetrahedral structures, whilst graphite's layered structure with weak forces between layers explains why you can write with pencil lead.
Visual Aid: Picture the nucleus as a magnet - more protons mean stronger pull on electrons, but more electron shells create a 'shielding' effect.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ionisation energy is the energy needed to remove an electron from an atom in the gas phase. It increases across a period because stronger nuclear charge holds electrons more tightly, but decreases down a group due to increased shielding from extra electron shells.
Elements in the bottom left of the periodic table have the lowest ionisation energies - they lose electrons most easily. This explains why Group 1 metals are so reactive and why they form positive ions readily.
Electronegativity measures how strongly an atom attracts bonding electrons. It follows the same trends as ionisation energy - increasing across periods and decreasing down groups. The values are given in your data booklet, so you don't need to memorise them.
The difference in electronegativity between bonded atoms determines bond type. Small differences (0.0-0.4) give non-polar covalent bonds with equal electron sharing. Larger differences create polar covalent bonds where electrons are pulled towards the more electronegative atom, creating partial charges .
Exam Tip: Always check if molecular shapes are symmetrical - even polar bonds can cancel out to give non-polar molecules overall!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Non-polar covalent bonding occurs when atoms have identical or very similar electronegativity values . Think F₂ or C-H bonds - electrons are shared equally between atoms, creating no partial charges.
Polar covalent bonding happens with moderate electronegativity differences. The more electronegative atom gets a partial negative charge (δ-) whilst the other becomes partially positive (δ+). Water's O-H bonds are perfect examples - oxygen pulls electrons away from hydrogen.
Here's the crucial bit: even molecules with polar bonds can be non-polar overall if they're symmetrical. CO₂ has polar C=O bonds, but because it's linear, the polarities cancel out. Always consider molecular shape when predicting polarity.
Ionic bonding forms when electronegativity differences are large (usually >1.7). Electrons transfer completely from metal to non-metal, creating charged ions held together by electrostatic attraction. Remember that metals typically form positive ions, non-metals form negative ones.
Memory Trick: Think of electronegativity like a tug-of-war - small difference means equal pull, big difference means one side wins completely!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The bonding continuum shows how bond types blend into each other based on electronegativity differences. Pure covalent (0.0-0.4) gradually becomes polar covalent, then ionic as differences increase. Don't expect sharp boundaries - chemistry loves gradual transitions.
Ionic compounds always conduct electricity when molten or dissolved because ions can move freely. Covalent compounds never conduct in any state because they don't have mobile charged particles. This difference is crucial for identifying compound types.
Van der Waals forces are weak intermolecular attractions that exist between all molecules. London dispersion forces (LDFs) are the weakest and occur everywhere due to temporary electron movement. More electrons mean stronger LDFs.
Permanent dipole-dipole interactions occur between polar molecules - the δ+ end of one molecule attracts the δ- end of another. Hydrogen bonding is the strongest intermolecular force, occurring when hydrogen bonds to F, O, or N (remember "FON").
Key Point: Intermolecular forces determine physical properties like boiling points - stronger forces mean higher temperatures needed to separate molecules.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
London dispersion forces exist in all substances and get stronger with more electrons. They're caused by temporary, uneven electron distribution creating temporary dipoles that attract neighbouring molecules.
Permanent dipole-dipole interactions occur between polar molecules where the positive end of one molecule attracts the negative end of another. These are stronger than LDFs but weaker than hydrogen bonds.
Hydrogen bonding only happens when hydrogen is directly bonded to fluorine, oxygen, or nitrogen. The small size and high electronegativity of these atoms create particularly strong dipoles. More -OH or -NH groups mean stronger hydrogen bonding overall.
The strength of intermolecular forces directly determines melting and boiling points. Propane (only LDFs) boils at -44°C, dimethyl ether at -22°C, but ethanol (hydrogen bonding) at +78°C. This pattern shows you can predict properties from molecular structure.
Practical Application: Understanding these forces explains why alcohol has a higher boiling point than expected for its size - it's all about those hydrogen bonds!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Remember "OIL RIG" - Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons). Oxidising agents cause other substances to be oxidised whilst getting reduced themselves. Reducing agents do the opposite - they cause reduction whilst being oxidised.
The electrochemical series ranks elements by how easily they lose electrons. Strong reducing agents (like lithium and potassium) appear at the top - they lose electrons readily due to low electronegativity. Strong oxidising agents (like fluorine and chlorine) appear at the bottom.
Group 1 metals make excellent reducing agents because they have low electronegativity and lose their outer electron easily. Group 7 elements are powerful oxidising agents because they readily gain electrons to complete their outer shells.
Common examples include hydrogen peroxide (H₂O₂) as a strong oxidising agent used in cleaning products and bleach, and carbon monoxide (CO) as a reducing agent. Oxidising agents kill bacteria and break down coloured compounds, explaining their use in disinfectants and bleaches.
Exam Strategy: When balancing redox equations, always balance elements first, then add H₂O for oxygen, H⁺ for hydrogen, and finally electrons for charge.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Hydrogen peroxide (H₂O₂) is a powerful oxidising agent you'll encounter frequently. It's used in cleaning products because it kills fungi and bacteria, and in bleaches because it breaks down coloured compounds by oxidising them.
Carbon monoxide (CO) serves as an important reducing agent, particularly in industrial processes like metal extraction where it removes oxygen from metal oxides.
Redox equations must balance both mass and charge. Start by balancing the main elements, then add H₂O molecules to balance oxygen atoms, H⁺ ions to balance hydrogen atoms, and finally electrons to balance the overall charge.
Displacement reactions follow predictable patterns using the electrochemical series. A more reactive metal (higher up the series) will displace a less reactive metal from its compounds. For example, zinc displaces silver from silver nitrate because zinc is higher in the series and more easily oxidised.
Real-world Connection: Understanding redox reactions explains everything from how batteries work to why iron rusts - it's oxidation and reduction happening all around us!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
7
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user