Ever wondered why nail varnish remover smells so strong, or... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
575
•
2 Feb 2026
•
Amy Neill
@amyneill
Ever wondered why nail varnish remover smells so strong, or... Show more





Welcome to Nature's Chemistry - where you'll discover how carbon atoms link together to create the incredible variety of compounds that surround us every day. This unit covers the building blocks of organic chemistry that you'll need for your exams.
From the petrol in cars to the alcohol in hand sanitiser, carbon compounds are everywhere. Understanding their patterns and behaviours will help you make sense of countless chemical reactions and properties.
Quick Tip: Think of carbon as nature's ultimate building block - it can form four bonds and create chains, rings, and complex structures that no other element can match!

A homologous series is basically a family of compounds that all behave similarly and follow the same general formula pattern. Think of them like different generations of the same family - they share key characteristics but get bigger as you go along.
Here are the main families you need to know: Alkanes (CₙH₂ₙ₊₂), Alkenes (CₙH₂ₙ), Cycloalkanes (CₙH₂ₙ), Alcohols (CₙH₂ₙ₊₁OH), and Carboxylic Acids (CₙH₂ₙ₊₁COOH). Each formula tells you exactly how many hydrogens you'll have for any number of carbons.
When naming branched hydrocarbons, follow these steps: find the longest carbon chain, identify any branches, number the carbons to give branches the lowest numbers, then list branches alphabetically. Use the memory trick "Meth- Eth- Prop- But- Pent- Hex- Hept- Oct" for carbon chain lengths.
Isomers are compounds with identical molecular formulas but different structural arrangements - like having the same Lego pieces but building different shapes.
Exam Alert: Always check your general formulas by substituting in small values of n - if CₙH₂ₙ₊₂ gives you CH₄ when n=1, you're on the right track!
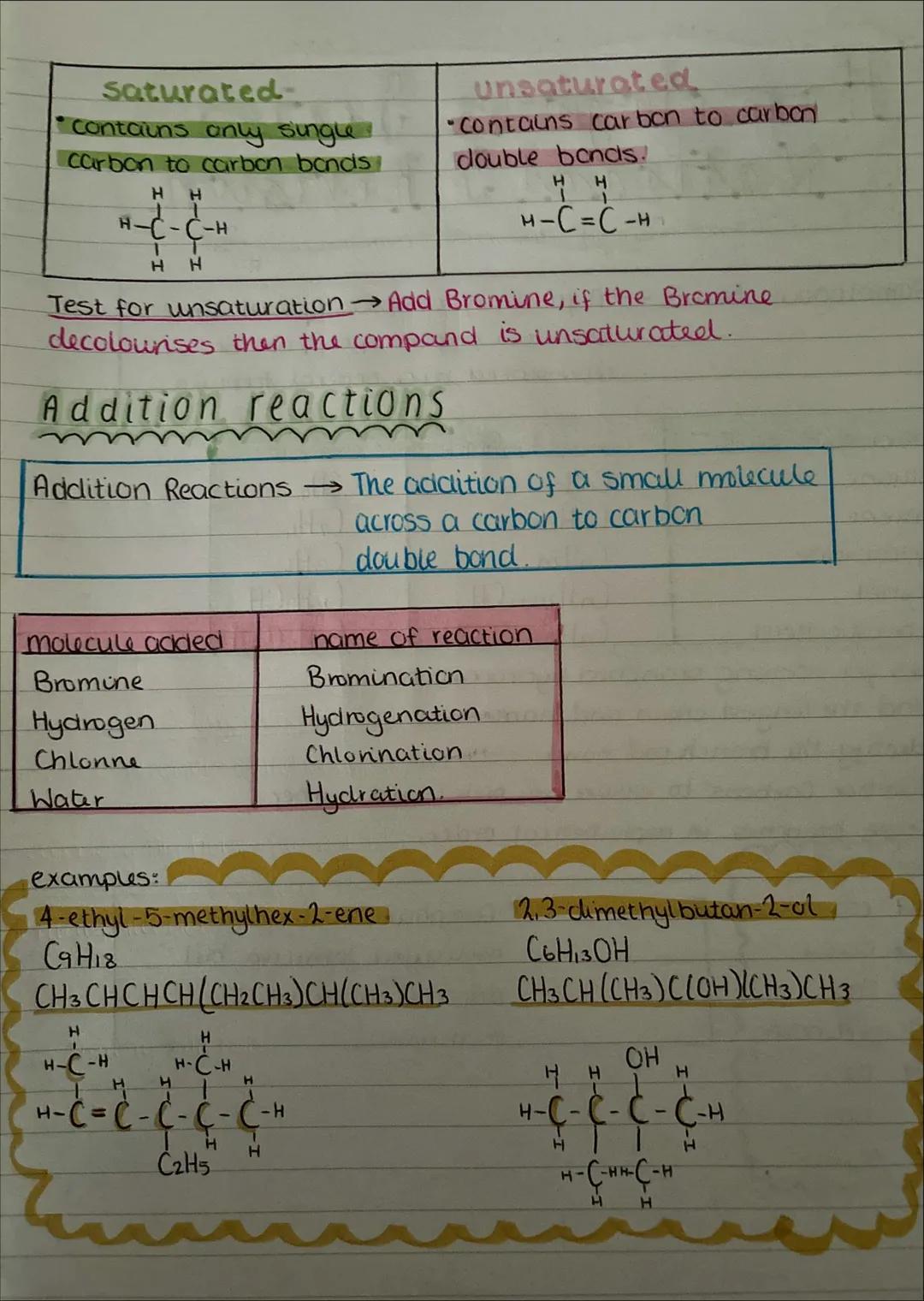
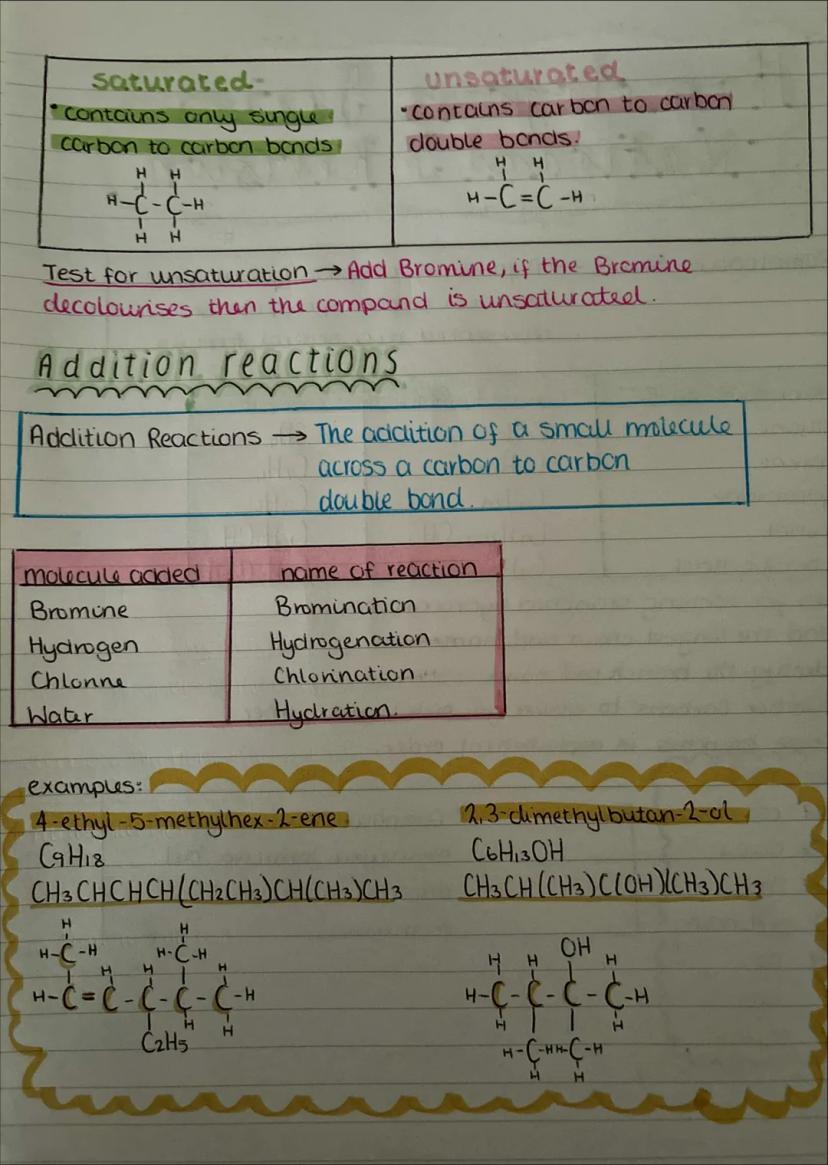
Saturated compounds contain only single carbon-carbon bonds, whilst unsaturated compounds have at least one carbon-carbon double bond. It's like the difference between a completely filled car park (saturated) and one with empty spaces (unsaturated).
The test for unsaturation is brilliantly simple: add bromine water to your compound. If it goes from orange/brown to colourless, you've got double bonds present. No colour change means it's saturated.
Addition reactions happen when small molecules add across double bonds. The main ones are bromination (adding Br₂), hydrogenation (adding H₂), chlorination (adding Cl₂), and hydration (adding H₂O). These reactions are crucial for making everything from margarine to plastics.
Real World: Hydrogenation is how manufacturers turn liquid vegetable oils into solid margarine - they're literally adding hydrogen across double bonds!
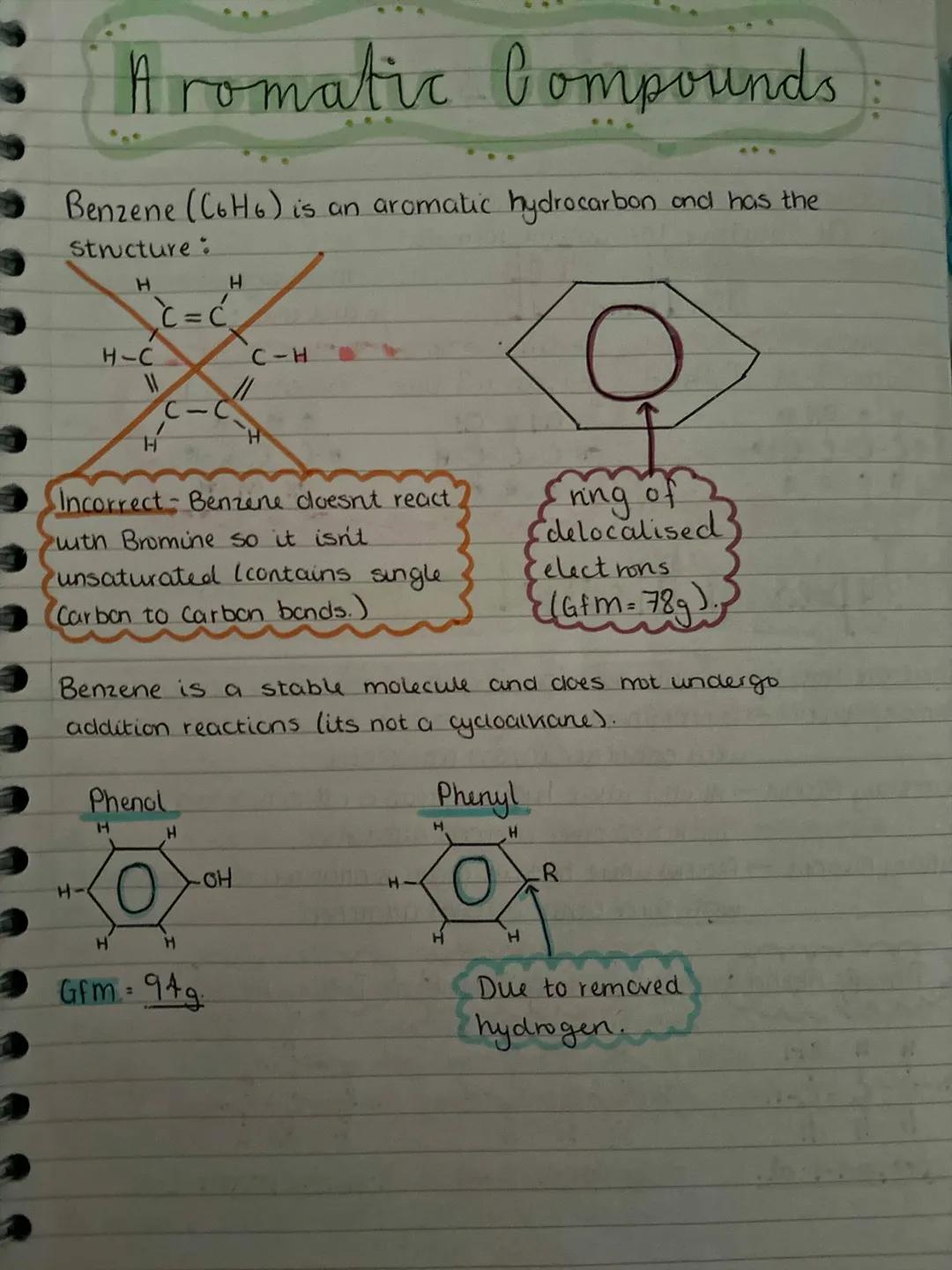
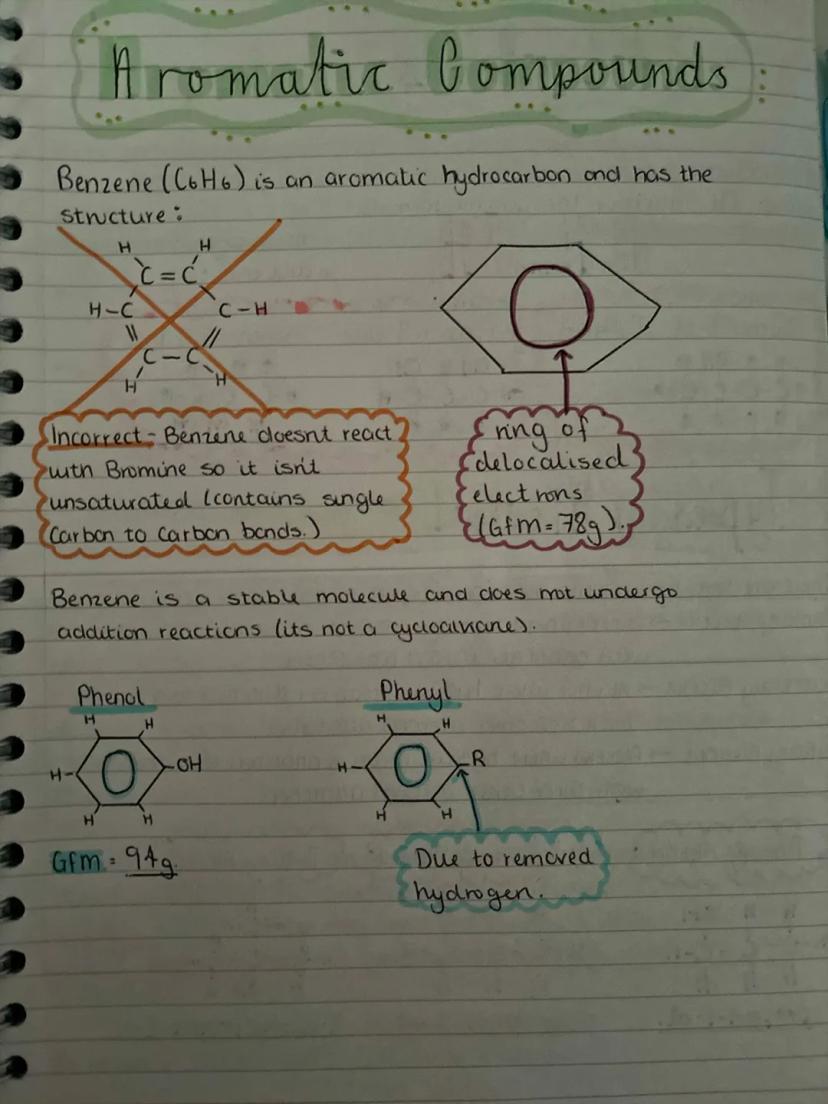
Benzene (C₆H₆) is the star of aromatic chemistry, but it's not what you might expect. Despite looking like it has double bonds, benzene doesn't react with bromine water - it's actually got a ring of delocalised electrons that makes it incredibly stable.
Don't be fooled by benzene's structure drawings. Those alternating single and double bonds are misleading - benzene is actually more stable than alkenes because its electrons are spread out around the ring. This is why it doesn't undergo addition reactions like alkenes do.
Phenol is benzene with an -OH group attached, whilst phenyl refers to benzene with a hydrogen removed (so it can attach to other groups). These aromatic compounds form the basis of countless important molecules, from aspirin to explosives.
Memory Trick: Think of benzene as the "aristocrat" of hydrocarbons - it's too stable and posh to react with just anyone!
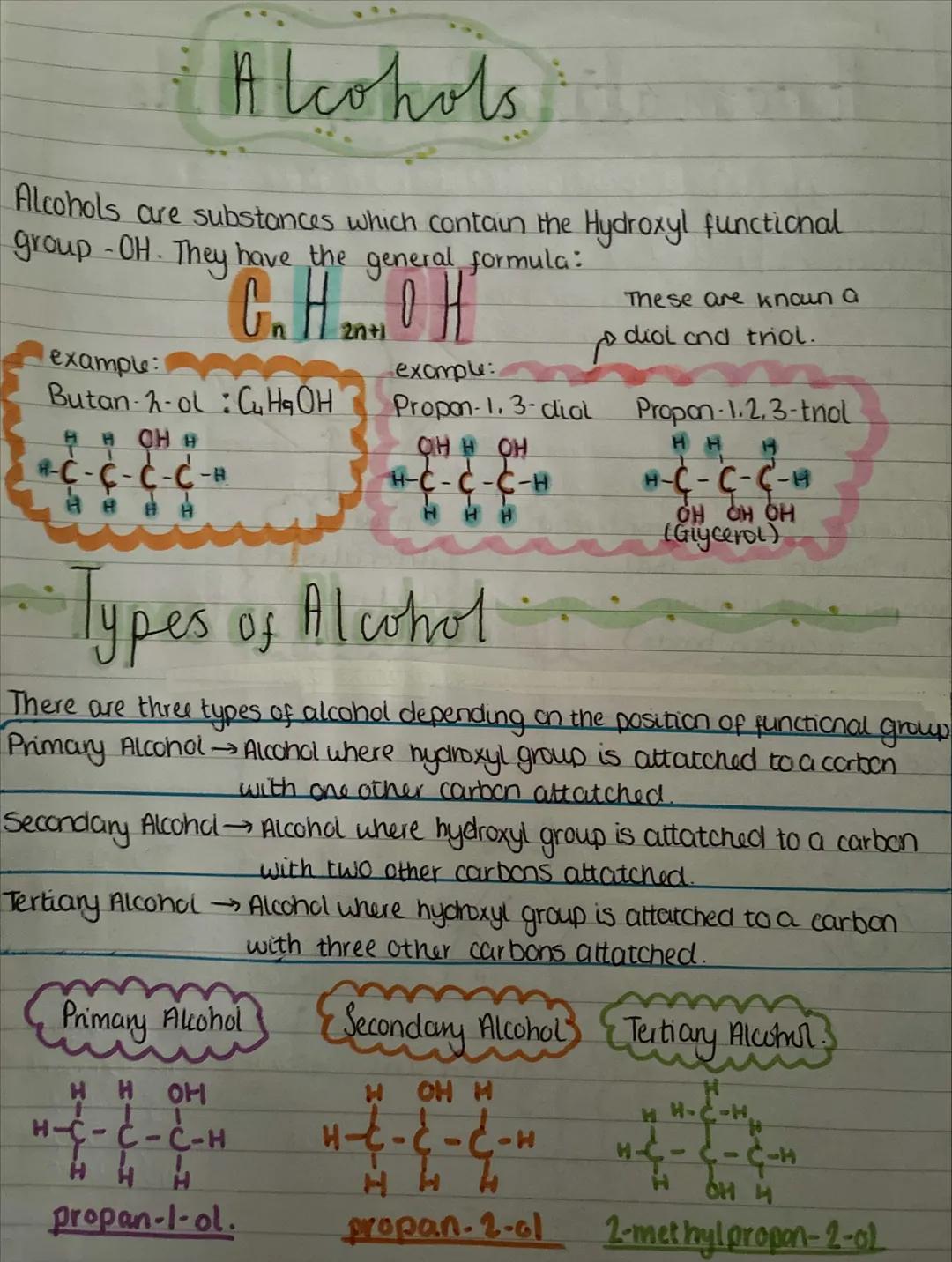
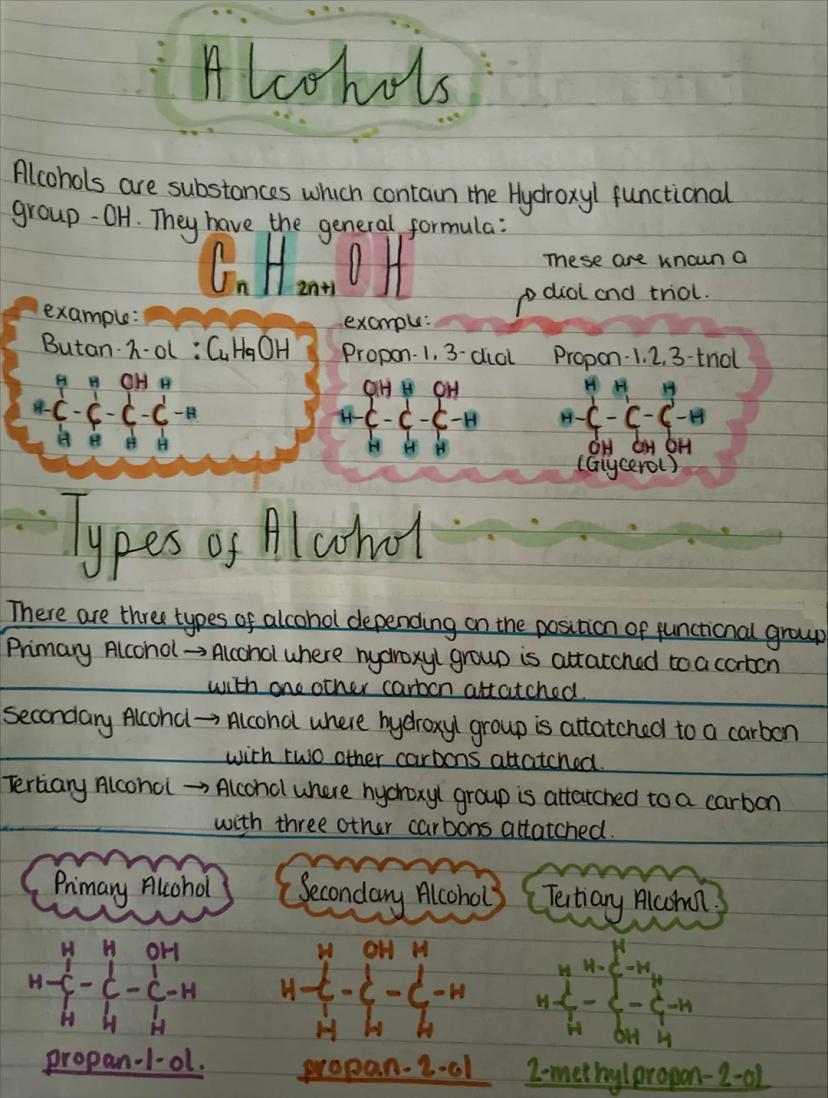
Alcohols contain the hydroxyl functional group and follow the general formula CₙH₂ₙ₊₁OH. When you've got multiple -OH groups, you get diols and triols like glycerol.
The position of the -OH group determines what type of alcohol you have. Primary alcohols have -OH attached to a carbon that's only connected to one other carbon. Secondary alcohols have -OH on a carbon connected to two other carbons. Tertiary alcohols have -OH on a carbon connected to three other carbons.
This classification isn't just academic - it affects how alcohols react. Primary alcohols like propan-1-ol behave differently from secondary ones like propan-2-ol, especially in oxidation reactions you'll meet later.
Visualisation Tip: Think of primary, secondary, and tertiary as describing how "crowded" the carbon with -OH is - one neighbour, two neighbours, or three neighbours!

Alcohols have much higher melting and boiling points than equivalent alkanes because they can form hydrogen bonds. These are stronger intermolecular forces than the simple London dispersion forces in alkanes.
Ethanol beats ethane in the boiling point stakes because ethanol molecules stick together through hydrogen bonding between the -OH groups. Ethane molecules only have weak London forces, so they separate much more easily.
Solubility follows similar rules - alcohols dissolve well in water because both are polar and can hydrogen bond together. Alkanes can't hydrogen bond with water, so they don't dissolve. As alcohol molecules get larger, they become less soluble because the non-polar carbon chain starts to dominate.
As molecular size increases, you'll see flammability decrease, solubility decrease, and melting points increase - these trends appear in loads of exam questions!
Real World: This is why spirits like vodka mix perfectly, but oil and water separate - it's all about those hydrogen bonds!
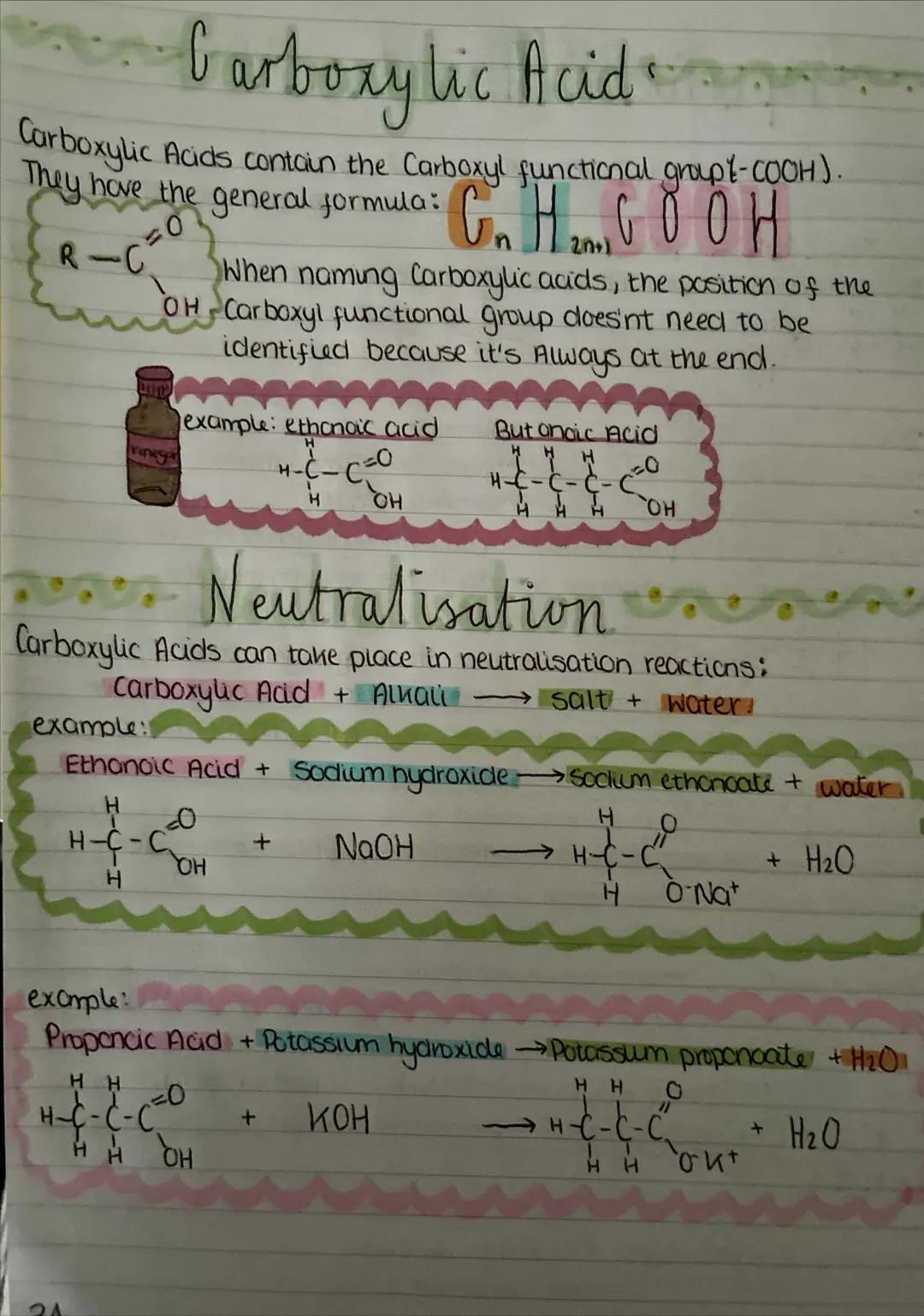
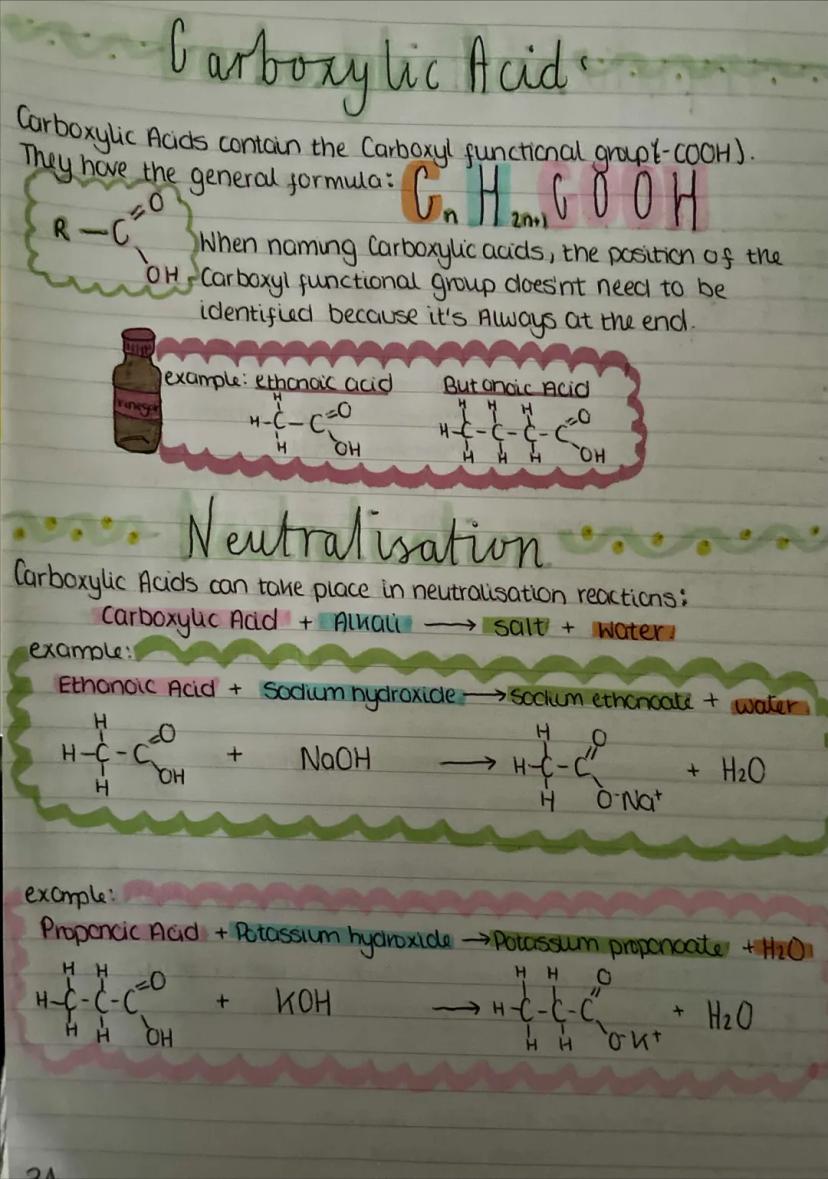
Carboxylic acids contain the carboxyl functional group and have the general formula CₙH₂ₙCOOH. The carboxyl group is always at the end of the molecule, so you don't need to number its position when naming.
Ethanoic acid is the acid in vinegar, whilst butanoic acid has that distinctive smell of rancid butter. These acids are everywhere in nature and industry.
Carboxylic acids undergo neutralisation reactions with alkalis to form salts and water. For example, ethanoic acid + sodium hydroxide → sodium ethanoate + water. This is the same pattern as any acid-base reaction, just with organic compounds.
The salt names follow a pattern: the metal comes from the alkali, and the acid name changes from "-oic acid" to "-oate". So propanoic acid with potassium hydroxide gives potassium propanoate.
Exam Tip: Remember that carboxylic acids are weak acids - they only partially ionise in solution, unlike strong acids like hydrochloric acid!

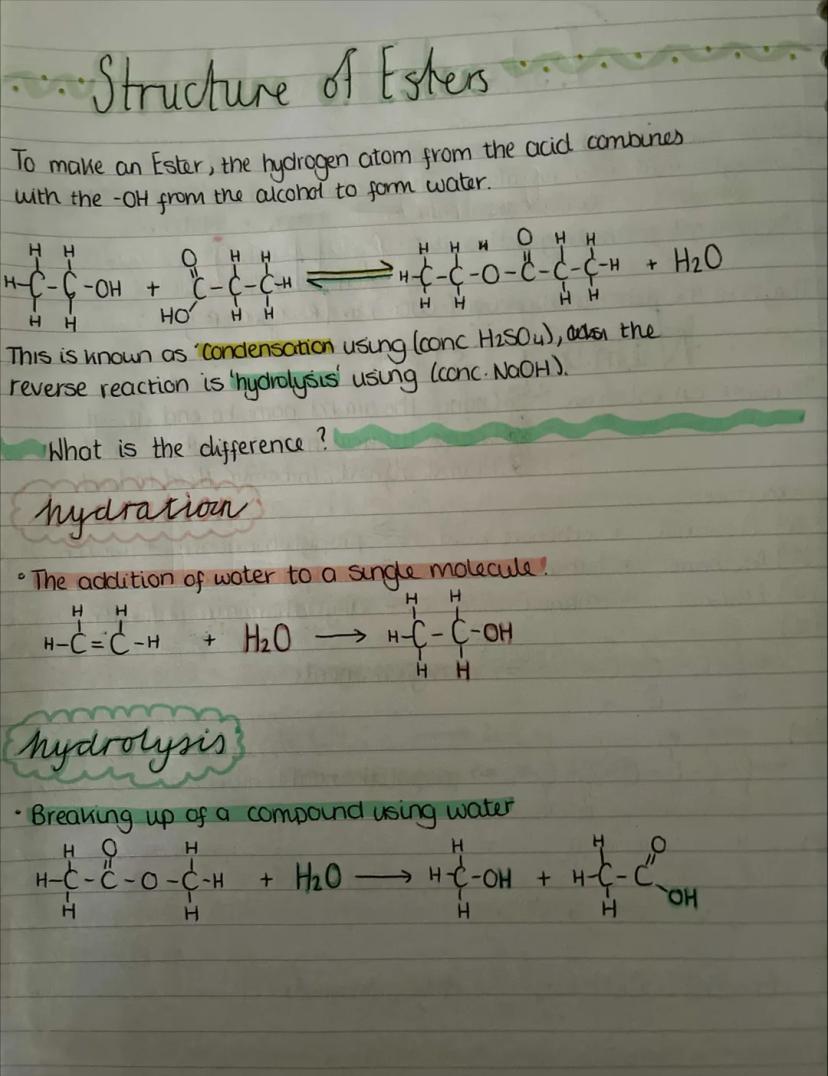
Esters form when a carboxylic acid reacts with an alcohol in a condensation reaction called esterification. The general formula is CₙH₂ₙO₂, and they contain the functional group -COO-.
Naming esters follows a specific pattern: change the alcohol name to end in "-yl", change the acid name to end in "-oate", then put alcohol first and acid second. So methanol + ethanoic acid gives methyl ethanoate.
Here's the key: propanol + ethanoic acid = propyl ethanoate, whilst ethanol + butanoic acid = ethyl butanoate. The alcohol part always comes first in the name, even though we often write the formula with the acid part first.
Esters are responsible for many fruity smells and flavours - ethyl ethanoate smells like pear drops, whilst other esters give bananas, apples, and oranges their characteristic aromas.
Memory Trick: Think "Alcohol Always first" - both start with A, so alcohol comes first in ester names!
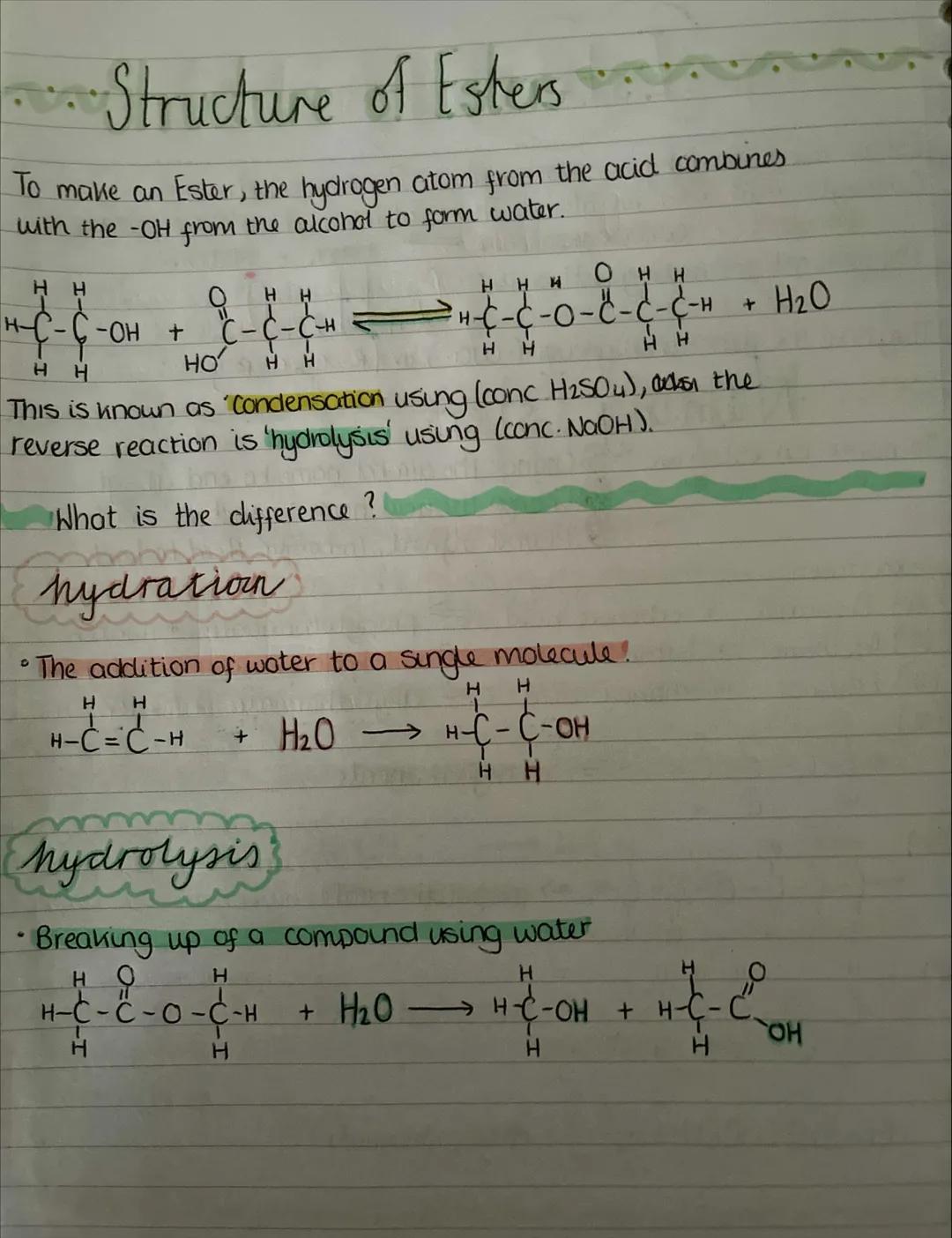
Esterification is a condensation reaction where the hydrogen from the carboxylic acid's -COOH group combines with the -OH from the alcohol to form water. What's left joins together to make the ester.
The reaction needs a concentrated sulfuric acid catalyst and is reversible. The reverse process is called hydrolysis - breaking the ester back down using water (usually with concentrated NaOH).
Don't confuse hydration and hydrolysis! Hydration adds water to a single molecule (like adding water across a double bond), whilst hydrolysis uses water to break apart a compound into two separate molecules.
This reversibility is crucial in biological systems - your body constantly makes and breaks esters in processes like fat metabolism and energy storage.
Quick Check: If you see water being formed in a reaction, it's condensation. If water is used up to break something apart, it's hydrolysis!
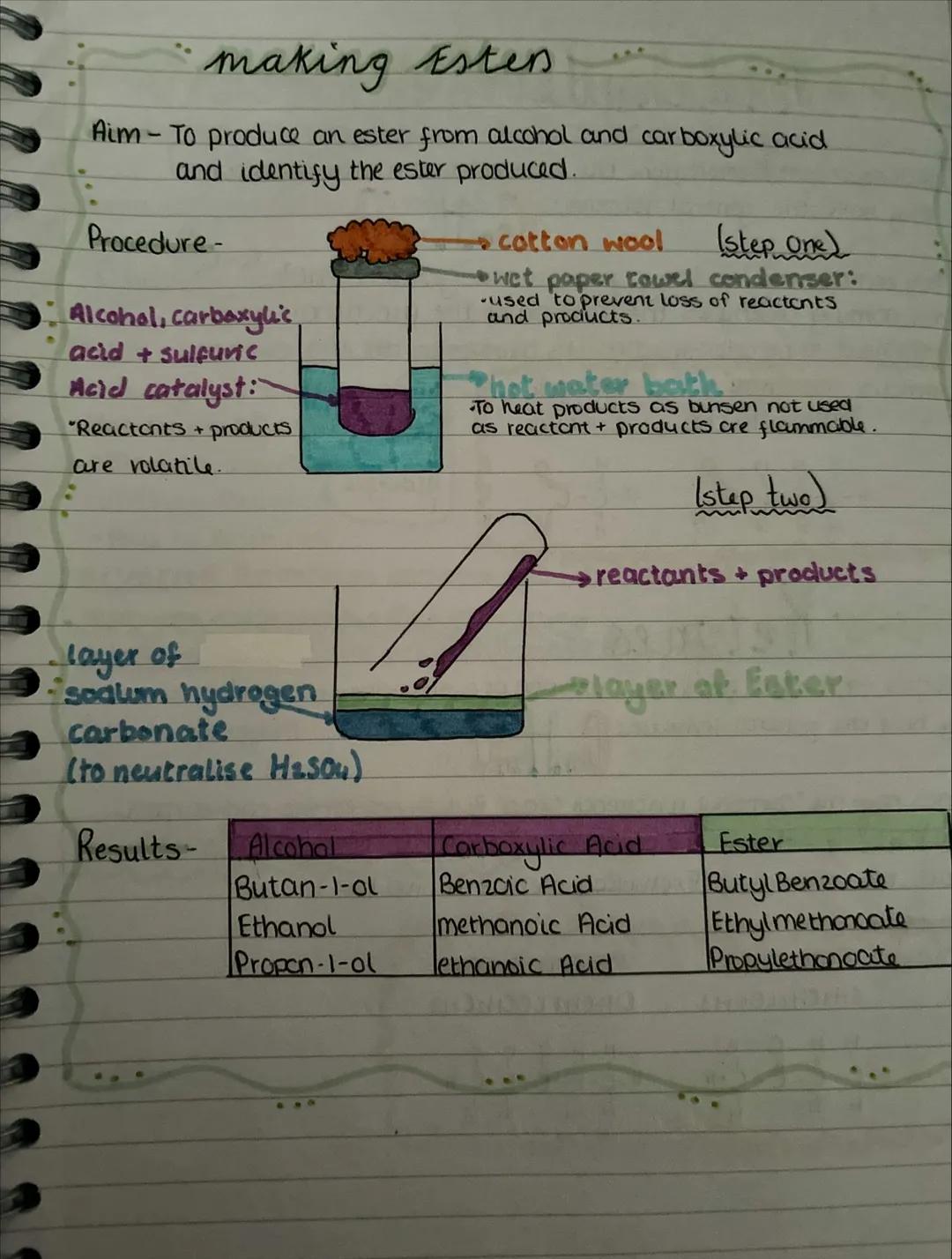
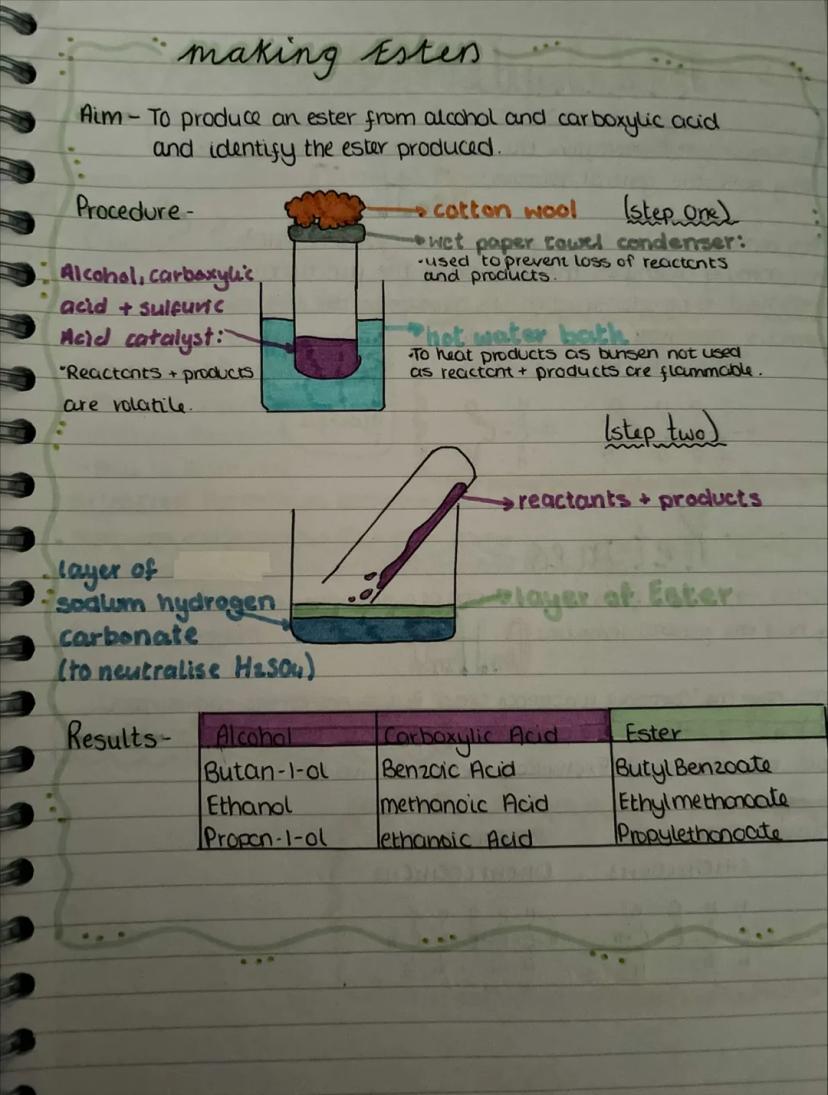
The practical for making esters involves mixing an alcohol, carboxylic acid, and concentrated sulfuric acid catalyst, then heating in a water bath. You can't use a Bunsen burner because the reactants and products are highly flammable.
A wet paper towel condenser prevents the volatile reactants and products from escaping - you need to keep everything in the reaction mixture for maximum ester formation.
After heating, you add the mixture to sodium hydrogen carbonate solution which neutralises the sulfuric acid catalyst. The ester forms a separate layer on top because it's less dense and doesn't dissolve well in the aqueous layer.
Common ester combinations: butan-1-ol + benzoic acid = butyl benzoate, ethanol + methanoic acid = ethyl methanoate, propan-1-ol + ethanoic acid = propyl ethanoate. Each has its own distinctive smell.
Safety Note: Always use a water bath for heating organic compounds - they're usually flammable and have low boiling points!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Amy Neill
@amyneill
Ever wondered why nail varnish remover smells so strong, or what gives fruits their distinctive aromas? It's all down to organic chemistry- the fascinating world of carbon-based compounds that make up everything from the fuel in your car to... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Welcome to Nature's Chemistry - where you'll discover how carbon atoms link together to create the incredible variety of compounds that surround us every day. This unit covers the building blocks of organic chemistry that you'll need for your exams.
From the petrol in cars to the alcohol in hand sanitiser, carbon compounds are everywhere. Understanding their patterns and behaviours will help you make sense of countless chemical reactions and properties.
Quick Tip: Think of carbon as nature's ultimate building block - it can form four bonds and create chains, rings, and complex structures that no other element can match!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
A homologous series is basically a family of compounds that all behave similarly and follow the same general formula pattern. Think of them like different generations of the same family - they share key characteristics but get bigger as you go along.
Here are the main families you need to know: Alkanes (CₙH₂ₙ₊₂), Alkenes (CₙH₂ₙ), Cycloalkanes (CₙH₂ₙ), Alcohols (CₙH₂ₙ₊₁OH), and Carboxylic Acids (CₙH₂ₙ₊₁COOH). Each formula tells you exactly how many hydrogens you'll have for any number of carbons.
When naming branched hydrocarbons, follow these steps: find the longest carbon chain, identify any branches, number the carbons to give branches the lowest numbers, then list branches alphabetically. Use the memory trick "Meth- Eth- Prop- But- Pent- Hex- Hept- Oct" for carbon chain lengths.
Isomers are compounds with identical molecular formulas but different structural arrangements - like having the same Lego pieces but building different shapes.
Exam Alert: Always check your general formulas by substituting in small values of n - if CₙH₂ₙ₊₂ gives you CH₄ when n=1, you're on the right track!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Saturated compounds contain only single carbon-carbon bonds, whilst unsaturated compounds have at least one carbon-carbon double bond. It's like the difference between a completely filled car park (saturated) and one with empty spaces (unsaturated).
The test for unsaturation is brilliantly simple: add bromine water to your compound. If it goes from orange/brown to colourless, you've got double bonds present. No colour change means it's saturated.
Addition reactions happen when small molecules add across double bonds. The main ones are bromination (adding Br₂), hydrogenation (adding H₂), chlorination (adding Cl₂), and hydration (adding H₂O). These reactions are crucial for making everything from margarine to plastics.
Real World: Hydrogenation is how manufacturers turn liquid vegetable oils into solid margarine - they're literally adding hydrogen across double bonds!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Benzene (C₆H₆) is the star of aromatic chemistry, but it's not what you might expect. Despite looking like it has double bonds, benzene doesn't react with bromine water - it's actually got a ring of delocalised electrons that makes it incredibly stable.
Don't be fooled by benzene's structure drawings. Those alternating single and double bonds are misleading - benzene is actually more stable than alkenes because its electrons are spread out around the ring. This is why it doesn't undergo addition reactions like alkenes do.
Phenol is benzene with an -OH group attached, whilst phenyl refers to benzene with a hydrogen removed (so it can attach to other groups). These aromatic compounds form the basis of countless important molecules, from aspirin to explosives.
Memory Trick: Think of benzene as the "aristocrat" of hydrocarbons - it's too stable and posh to react with just anyone!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Alcohols contain the hydroxyl functional group and follow the general formula CₙH₂ₙ₊₁OH. When you've got multiple -OH groups, you get diols and triols like glycerol.
The position of the -OH group determines what type of alcohol you have. Primary alcohols have -OH attached to a carbon that's only connected to one other carbon. Secondary alcohols have -OH on a carbon connected to two other carbons. Tertiary alcohols have -OH on a carbon connected to three other carbons.
This classification isn't just academic - it affects how alcohols react. Primary alcohols like propan-1-ol behave differently from secondary ones like propan-2-ol, especially in oxidation reactions you'll meet later.
Visualisation Tip: Think of primary, secondary, and tertiary as describing how "crowded" the carbon with -OH is - one neighbour, two neighbours, or three neighbours!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Alcohols have much higher melting and boiling points than equivalent alkanes because they can form hydrogen bonds. These are stronger intermolecular forces than the simple London dispersion forces in alkanes.
Ethanol beats ethane in the boiling point stakes because ethanol molecules stick together through hydrogen bonding between the -OH groups. Ethane molecules only have weak London forces, so they separate much more easily.
Solubility follows similar rules - alcohols dissolve well in water because both are polar and can hydrogen bond together. Alkanes can't hydrogen bond with water, so they don't dissolve. As alcohol molecules get larger, they become less soluble because the non-polar carbon chain starts to dominate.
As molecular size increases, you'll see flammability decrease, solubility decrease, and melting points increase - these trends appear in loads of exam questions!
Real World: This is why spirits like vodka mix perfectly, but oil and water separate - it's all about those hydrogen bonds!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Carboxylic acids contain the carboxyl functional group and have the general formula CₙH₂ₙCOOH. The carboxyl group is always at the end of the molecule, so you don't need to number its position when naming.
Ethanoic acid is the acid in vinegar, whilst butanoic acid has that distinctive smell of rancid butter. These acids are everywhere in nature and industry.
Carboxylic acids undergo neutralisation reactions with alkalis to form salts and water. For example, ethanoic acid + sodium hydroxide → sodium ethanoate + water. This is the same pattern as any acid-base reaction, just with organic compounds.
The salt names follow a pattern: the metal comes from the alkali, and the acid name changes from "-oic acid" to "-oate". So propanoic acid with potassium hydroxide gives potassium propanoate.
Exam Tip: Remember that carboxylic acids are weak acids - they only partially ionise in solution, unlike strong acids like hydrochloric acid!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Esters form when a carboxylic acid reacts with an alcohol in a condensation reaction called esterification. The general formula is CₙH₂ₙO₂, and they contain the functional group -COO-.
Naming esters follows a specific pattern: change the alcohol name to end in "-yl", change the acid name to end in "-oate", then put alcohol first and acid second. So methanol + ethanoic acid gives methyl ethanoate.
Here's the key: propanol + ethanoic acid = propyl ethanoate, whilst ethanol + butanoic acid = ethyl butanoate. The alcohol part always comes first in the name, even though we often write the formula with the acid part first.
Esters are responsible for many fruity smells and flavours - ethyl ethanoate smells like pear drops, whilst other esters give bananas, apples, and oranges their characteristic aromas.
Memory Trick: Think "Alcohol Always first" - both start with A, so alcohol comes first in ester names!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Esterification is a condensation reaction where the hydrogen from the carboxylic acid's -COOH group combines with the -OH from the alcohol to form water. What's left joins together to make the ester.
The reaction needs a concentrated sulfuric acid catalyst and is reversible. The reverse process is called hydrolysis - breaking the ester back down using water (usually with concentrated NaOH).
Don't confuse hydration and hydrolysis! Hydration adds water to a single molecule (like adding water across a double bond), whilst hydrolysis uses water to break apart a compound into two separate molecules.
This reversibility is crucial in biological systems - your body constantly makes and breaks esters in processes like fat metabolism and energy storage.
Quick Check: If you see water being formed in a reaction, it's condensation. If water is used up to break something apart, it's hydrolysis!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The practical for making esters involves mixing an alcohol, carboxylic acid, and concentrated sulfuric acid catalyst, then heating in a water bath. You can't use a Bunsen burner because the reactants and products are highly flammable.
A wet paper towel condenser prevents the volatile reactants and products from escaping - you need to keep everything in the reaction mixture for maximum ester formation.
After heating, you add the mixture to sodium hydrogen carbonate solution which neutralises the sulfuric acid catalyst. The ester forms a separate layer on top because it's less dense and doesn't dissolve well in the aqueous layer.
Common ester combinations: butan-1-ol + benzoic acid = butyl benzoate, ethanol + methanoic acid = ethyl methanoate, propan-1-ol + ethanoic acid = propyl ethanoate. Each has its own distinctive smell.
Safety Note: Always use a water bath for heating organic compounds - they're usually flammable and have low boiling points!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
19
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the fundamentals of redox reactions, including oxidation and reduction processes, oxidation states, and half-equations. This summary provides clear definitions and rules for identifying oxidizing and reducing agents, making it an essential resource for AQA AS Physical Chemistry students.
Explore key concepts in organic chemistry, including esters, carboxylic acids, oxidation-reduction reactions, and the chemistry of fats and proteins. This comprehensive summary covers essential topics such as saponification, emulsifiers, and the role of functional groups in molecular structure. Ideal for students preparing for higher chemistry exams.
Explore the fundamentals of redox reactions, including oxidation and reduction processes, and the role of acids and bases in chemical reactions. This summary covers key concepts such as oxidizing and reducing agents, acid-base neutralization, and titration techniques, essential for A-Level Chemistry students.
Explore a comprehensive flowchart detailing the mechanisms of organic synthesis, including reactions involving esters, nitriles, alkanes, and more. This resource covers key concepts such as acid-base catalysis, nucleophilic substitution, and electrophilic addition, making it essential for students studying organic chemistry. Ideal for exam preparation and understanding complex reaction pathways.
Explore essential chemistry terms and definitions, including aldehydes, ketones, intermolecular forces, and titration techniques. This summary provides clear explanations of key concepts such as percent yield, atom economy, and the role of hydrogen bonds in chemical reactions. Ideal for students preparing for exams or seeking to enhance their understanding of higher chemistry topics.
Explore the fundamentals of chemical bonding, including ionic, covalent, and metallic bonds. Understand bond polarity, molecular geometry, and intermolecular forces such as hydrogen bonding and van der Waals forces. This summary covers key concepts like electronegativity, VSEPR theory, and the properties of various bonding types, making it essential for AQA A-Level Chemistry students.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user