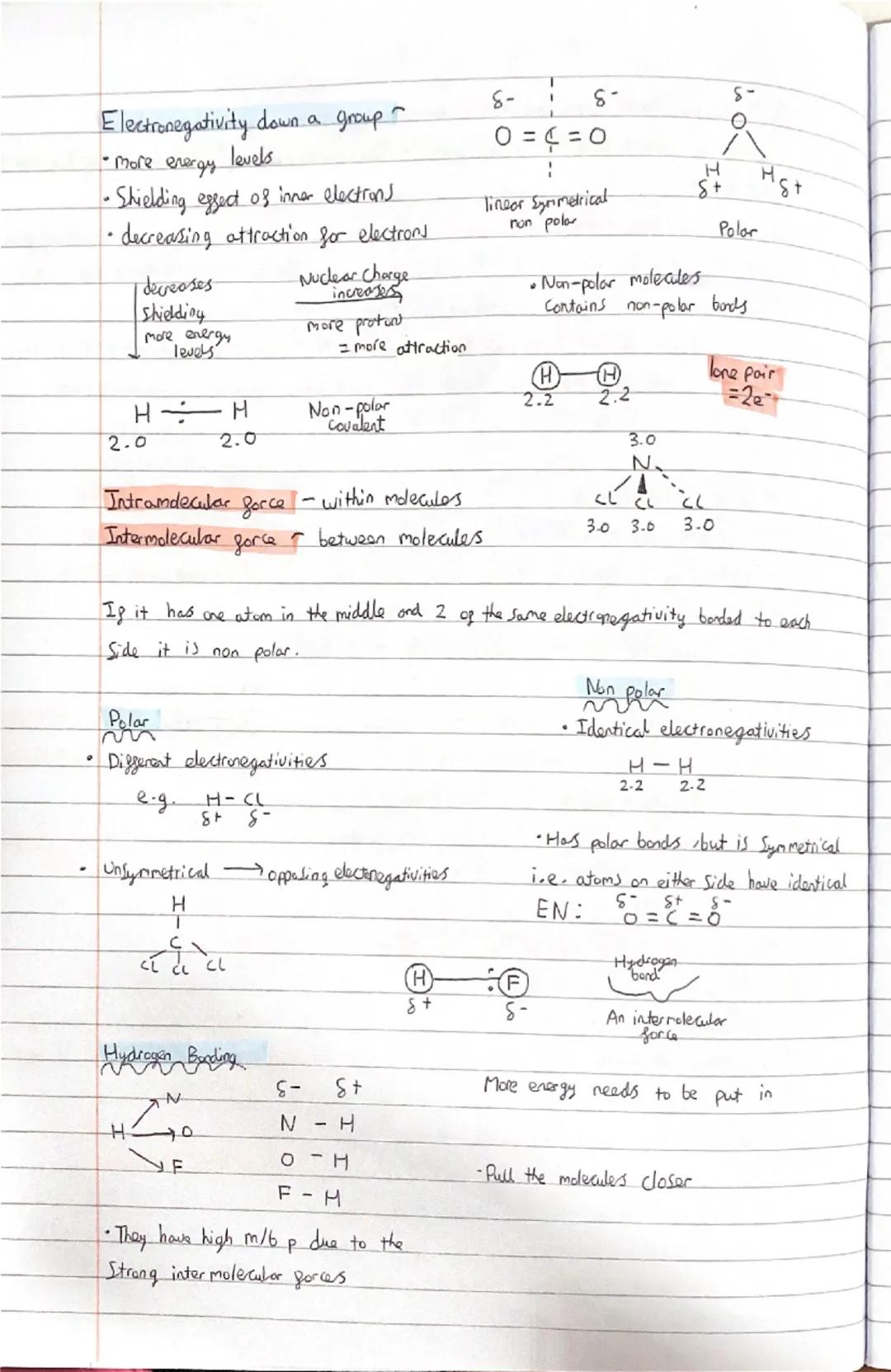
Ionisation Energy and Atomic Structure
Ionisation energy - the energy needed to remove one mole of electrons from gaseous atoms - follows the same pattern as electronegativity. You might need to write equations for first, second, or third ionisation energies, so check page 123 of your data booklet.
The trend makes perfect sense when you think about it. Going down groups, outer electrons are further from the nucleus with more inner electrons providing a screening effect, so less energy removes them. Across periods, increasing nuclear charge means electrons are held more tightly.
Atomic size changes are driven by two competing factors: nuclear charge (pulls electrons closer) versus number of energy levels (pushes them further out). Across periods, nuclear charge wins. Down groups, extra energy levels dominate.
Remember: The nuclear charge increase across periods affects all these trends - it's the driving force behind most periodic patterns.