Ever wonder how scientists figured out what atoms actually look... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
86
•
7 Feb 2026
•
Zainab Adetoun
@ainabdetoun_fabahyqf
Ever wonder how scientists figured out what atoms actually look... Show more








Chemical equations are like recipes for reactions - they show exactly what goes in and what comes out. Reactants (the starting materials) appear before the arrow, whilst products (what you end up with) come after it.
The golden rule is that equations must be balanced - you need the same number of each type of atom on both sides. Think of it like a see-saw that needs to be perfectly level. To balance equations, add numbers in front of the chemical formulas, but never change the formulas themselves.
Here's how to tackle balancing: Start with magnesium + oxygen → magnesium oxide, which becomes Mg + O₂ → MgO. First, balance the oxygen by adding a 2 in front of MgO, giving you Mg + O₂ → 2MgO. Then balance the magnesium by adding a 2 in front of Mg, resulting in 2Mg + O₂ → 2MgO.
Top Tip: Count atoms of each element separately and balance one at a time - trying to do everything at once will leave you confused!
State symbols tell you the physical state of each substance in a reaction. You'll use (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). These symbols go in brackets right after each chemical formula.
A complete equation with state symbols looks like this: 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g). This shows solid sodium reacting with liquid water to produce an aqueous solution and hydrogen gas.
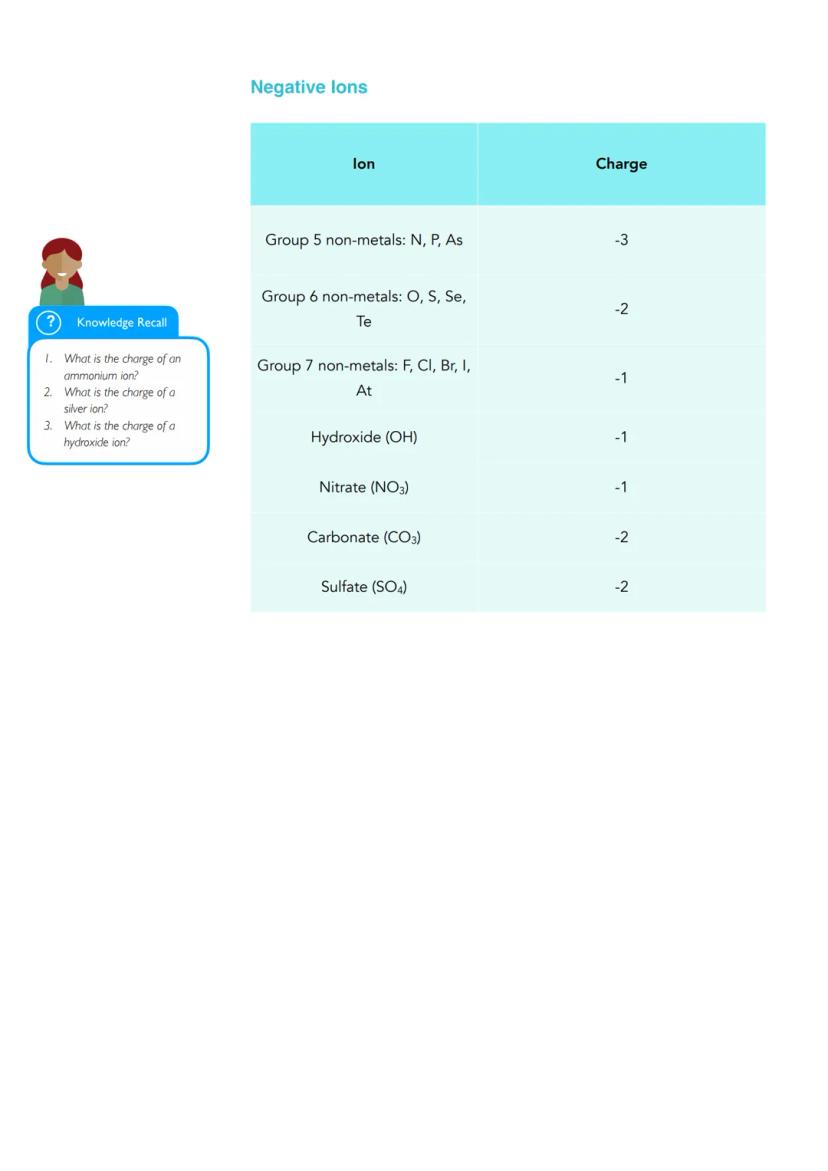
Common ions have predictable charges based on their position in the periodic table. Group 1 metals like sodium always have a +1 charge, whilst Group 2 metals like magnesium have +2. Important negative ions include hydroxide (OH⁻), nitrate (NO₃⁻), carbonate (CO₃²⁻), and sulfate (SO₄²⁻).
Remember: Aqueous simply means "dissolved in water" - so when you see (aq), think of a solution you could actually pour!

Everything around you is made of atoms - the smallest possible units of matter that can exist. When atoms of the same type group together, they form elements, like pure oxygen or carbon. Elements are organised in the periodic table by atomic number.
Compounds form when two or more different elements chemically combine in fixed proportions. Water (H₂O) always has exactly 2 hydrogen atoms for every oxygen atom - never more, never less. This is completely different from just mixing elements together.
Creating compounds involves making and breaking chemical bonds through electron sharing, transfer, or rearrangement. This process usually releases or requires energy, which is why some reactions feel hot whilst others feel cold. Covalent bonds form when electrons are shared, whilst ionic bonds form when electrons transfer from metals to non-metals.
Key Point: The difference between elements and compounds is fundamental to all of chemistry - make sure you've got this distinction crystal clear!

Scientists' understanding of atoms has evolved dramatically through experiments and evidence. The journey began with John Dalton's Billiard Ball Model in the early 1800s, which suggested atoms were solid, indivisible spheres that could be rearranged during chemical reactions.
Thompson's discovery of electrons completely revolutionised atomic theory. His Plum Pudding Model proposed that atoms were spheres of positive charge with negatively charged electrons embedded randomly throughout, like plums in a pudding. The mass was thought to be evenly distributed across the entire atom.
This model seemed logical at the time - it explained why atoms were generally neutral (positive and negative charges balanced out) and accounted for the newly discovered electrons. However, it was about to be completely overturned by a famous experiment.
Study Smart: Learn each model's key features systematically - exam questions love testing the differences between these early theories!

Rutherford's alpha particle scattering experiment changed everything we thought we knew about atoms. He fired tiny, positively charged alpha particles at extremely thin gold foil, expecting them all to pass straight through based on the plum pudding model.
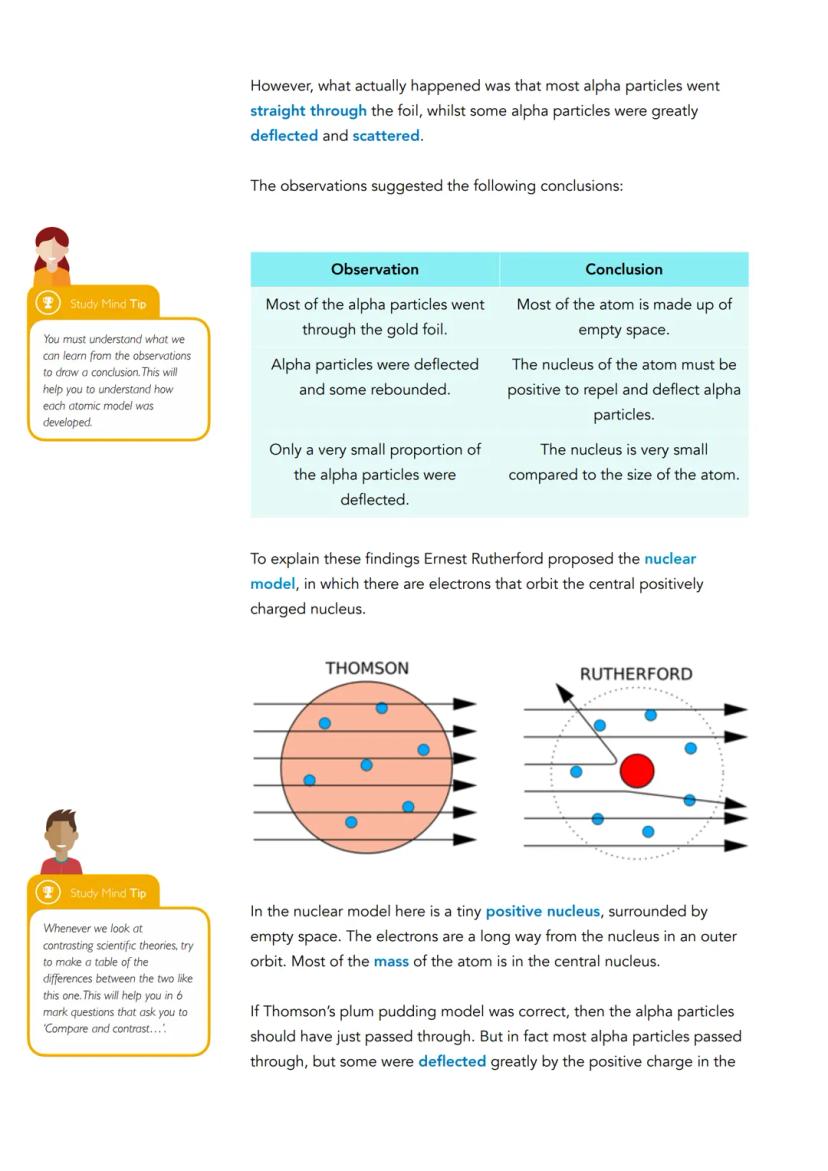
The results were shocking. Whilst most alpha particles did pass through undeflected, some were dramatically deflected or even bounced back completely. This was like firing bullets at tissue paper and having some bounce back at you!
These observations led to three crucial conclusions: most of the atom is empty space (explaining why most particles passed through), the nucleus must be positively charged (to repel the positive alpha particles), and the nucleus is incredibly tiny compared to the atom's total size (since only a few particles were deflected).
Rutherford's Nuclear Model placed a tiny, dense, positive nucleus at the atom's centre, with electrons orbiting in the surrounding empty space - completely revolutionising atomic theory.
Exam Focus: Understanding what each observation proved is crucial - questions often ask you to link specific results to conclusions about atomic structure!





Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Zainab Adetoun
@ainabdetoun_fabahyqf
Ever wonder how scientists figured out what atoms actually look like or why chemical equations need to balance? This guide breaks down the essential chemistry concepts you'll need to master - from writing balanced equations to understanding how our model... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
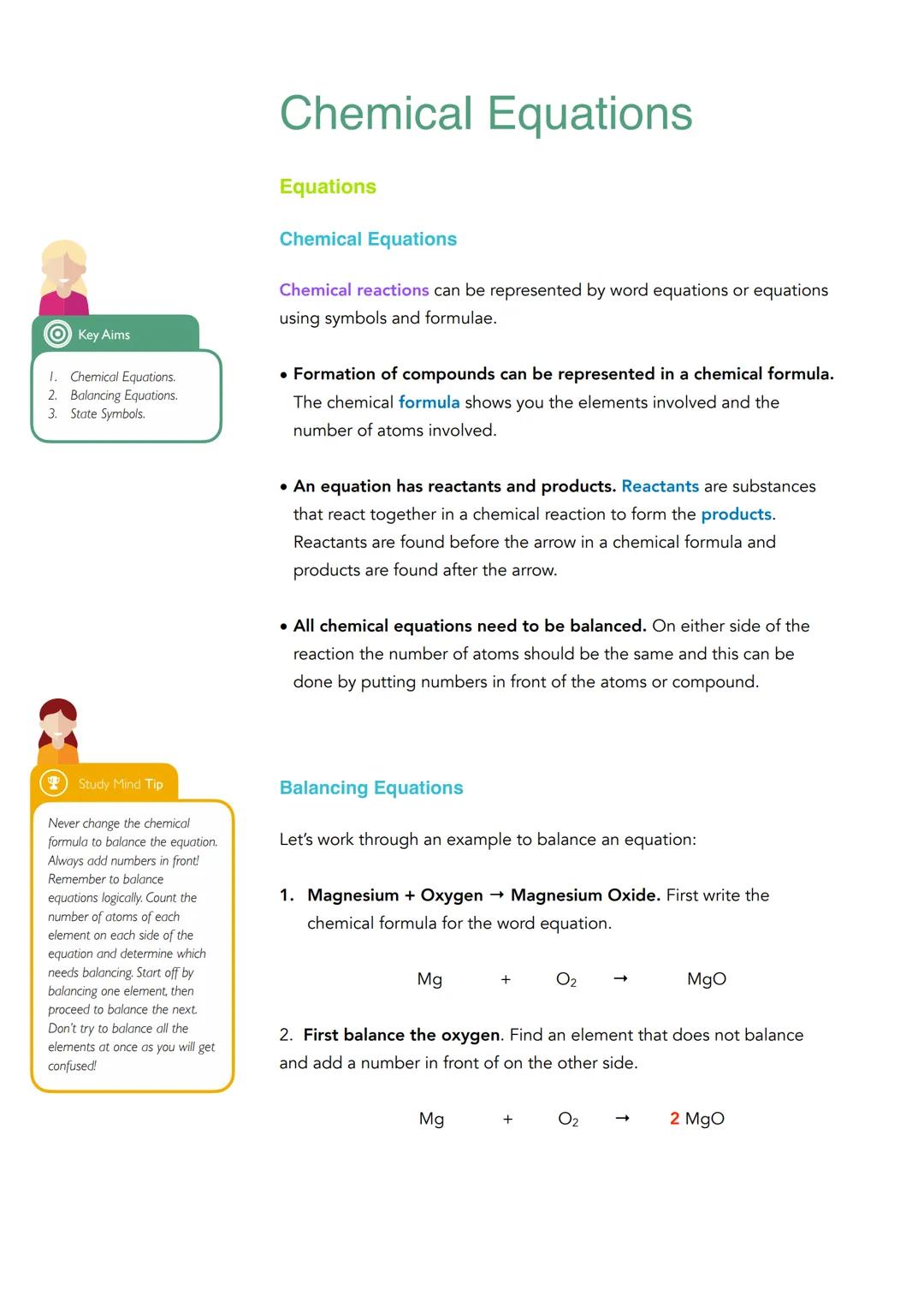
Chemical equations are like recipes for reactions - they show exactly what goes in and what comes out. Reactants (the starting materials) appear before the arrow, whilst products (what you end up with) come after it.
The golden rule is that equations must be balanced - you need the same number of each type of atom on both sides. Think of it like a see-saw that needs to be perfectly level. To balance equations, add numbers in front of the chemical formulas, but never change the formulas themselves.
Here's how to tackle balancing: Start with magnesium + oxygen → magnesium oxide, which becomes Mg + O₂ → MgO. First, balance the oxygen by adding a 2 in front of MgO, giving you Mg + O₂ → 2MgO. Then balance the magnesium by adding a 2 in front of Mg, resulting in 2Mg + O₂ → 2MgO.
Top Tip: Count atoms of each element separately and balance one at a time - trying to do everything at once will leave you confused!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
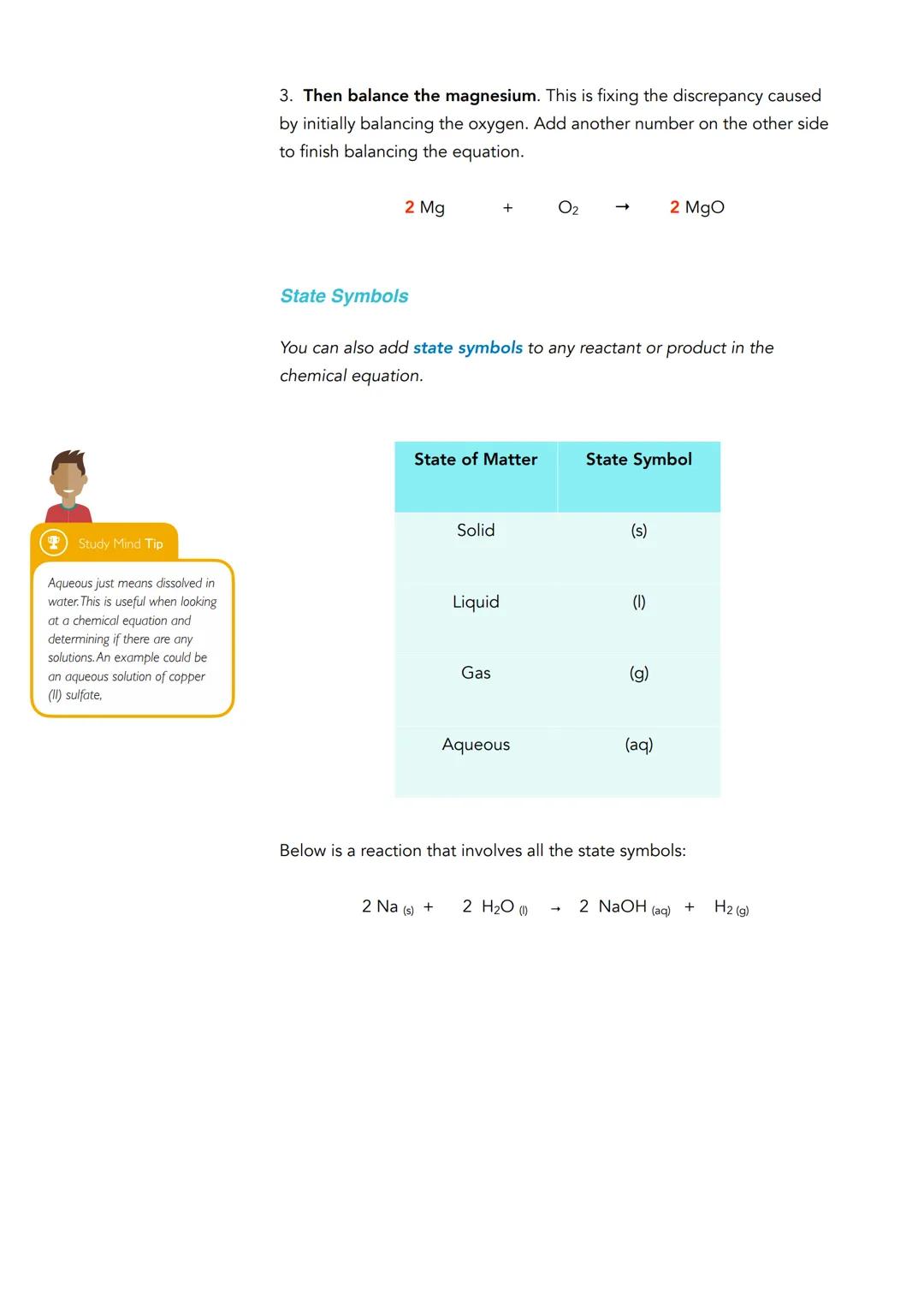
State symbols tell you the physical state of each substance in a reaction. You'll use (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). These symbols go in brackets right after each chemical formula.
A complete equation with state symbols looks like this: 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g). This shows solid sodium reacting with liquid water to produce an aqueous solution and hydrogen gas.
Common ions have predictable charges based on their position in the periodic table. Group 1 metals like sodium always have a +1 charge, whilst Group 2 metals like magnesium have +2. Important negative ions include hydroxide (OH⁻), nitrate (NO₃⁻), carbonate (CO₃²⁻), and sulfate (SO₄²⁻).
Remember: Aqueous simply means "dissolved in water" - so when you see (aq), think of a solution you could actually pour!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Everything around you is made of atoms - the smallest possible units of matter that can exist. When atoms of the same type group together, they form elements, like pure oxygen or carbon. Elements are organised in the periodic table by atomic number.
Compounds form when two or more different elements chemically combine in fixed proportions. Water (H₂O) always has exactly 2 hydrogen atoms for every oxygen atom - never more, never less. This is completely different from just mixing elements together.
Creating compounds involves making and breaking chemical bonds through electron sharing, transfer, or rearrangement. This process usually releases or requires energy, which is why some reactions feel hot whilst others feel cold. Covalent bonds form when electrons are shared, whilst ionic bonds form when electrons transfer from metals to non-metals.
Key Point: The difference between elements and compounds is fundamental to all of chemistry - make sure you've got this distinction crystal clear!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Scientists' understanding of atoms has evolved dramatically through experiments and evidence. The journey began with John Dalton's Billiard Ball Model in the early 1800s, which suggested atoms were solid, indivisible spheres that could be rearranged during chemical reactions.
Thompson's discovery of electrons completely revolutionised atomic theory. His Plum Pudding Model proposed that atoms were spheres of positive charge with negatively charged electrons embedded randomly throughout, like plums in a pudding. The mass was thought to be evenly distributed across the entire atom.
This model seemed logical at the time - it explained why atoms were generally neutral (positive and negative charges balanced out) and accounted for the newly discovered electrons. However, it was about to be completely overturned by a famous experiment.
Study Smart: Learn each model's key features systematically - exam questions love testing the differences between these early theories!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Rutherford's alpha particle scattering experiment changed everything we thought we knew about atoms. He fired tiny, positively charged alpha particles at extremely thin gold foil, expecting them all to pass straight through based on the plum pudding model.
The results were shocking. Whilst most alpha particles did pass through undeflected, some were dramatically deflected or even bounced back completely. This was like firing bullets at tissue paper and having some bounce back at you!
These observations led to three crucial conclusions: most of the atom is empty space (explaining why most particles passed through), the nucleus must be positively charged (to repel the positive alpha particles), and the nucleus is incredibly tiny compared to the atom's total size (since only a few particles were deflected).
Rutherford's Nuclear Model placed a tiny, dense, positive nucleus at the atom's centre, with electrons orbiting in the surrounding empty space - completely revolutionising atomic theory.
Exam Focus: Understanding what each observation proved is crucial - questions often ask you to link specific results to conclusions about atomic structure!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
2
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user