Ever wondered what makes up everything around you? Atoms are... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
193
•
13 Feb 2026
•
defne
@defneguvenn
Ever wondered what makes up everything around you? Atoms are... Show more






Everything you touch, breathe, or see is made up of incredibly tiny particles called atoms. Think of them as nature's LEGO blocks - they're the smallest parts of an element that still keep all its properties.
Elements are pure substances made from just one type of atom. You'll find all known elements organised on the periodic table, each with its own symbol (like H for hydrogen or O for oxygen). The periodic table isn't random - it's arranged in columns called groups and rows called periods for good reason.
Elements in the same group have similar chemical properties, which means they behave alike in reactions. The period an element sits in tells you how many electron shells its atoms have - pretty neat, right?
Quick tip: The periodic table is like a cheat sheet for chemistry - learn to read it and you'll save loads of time in exams!
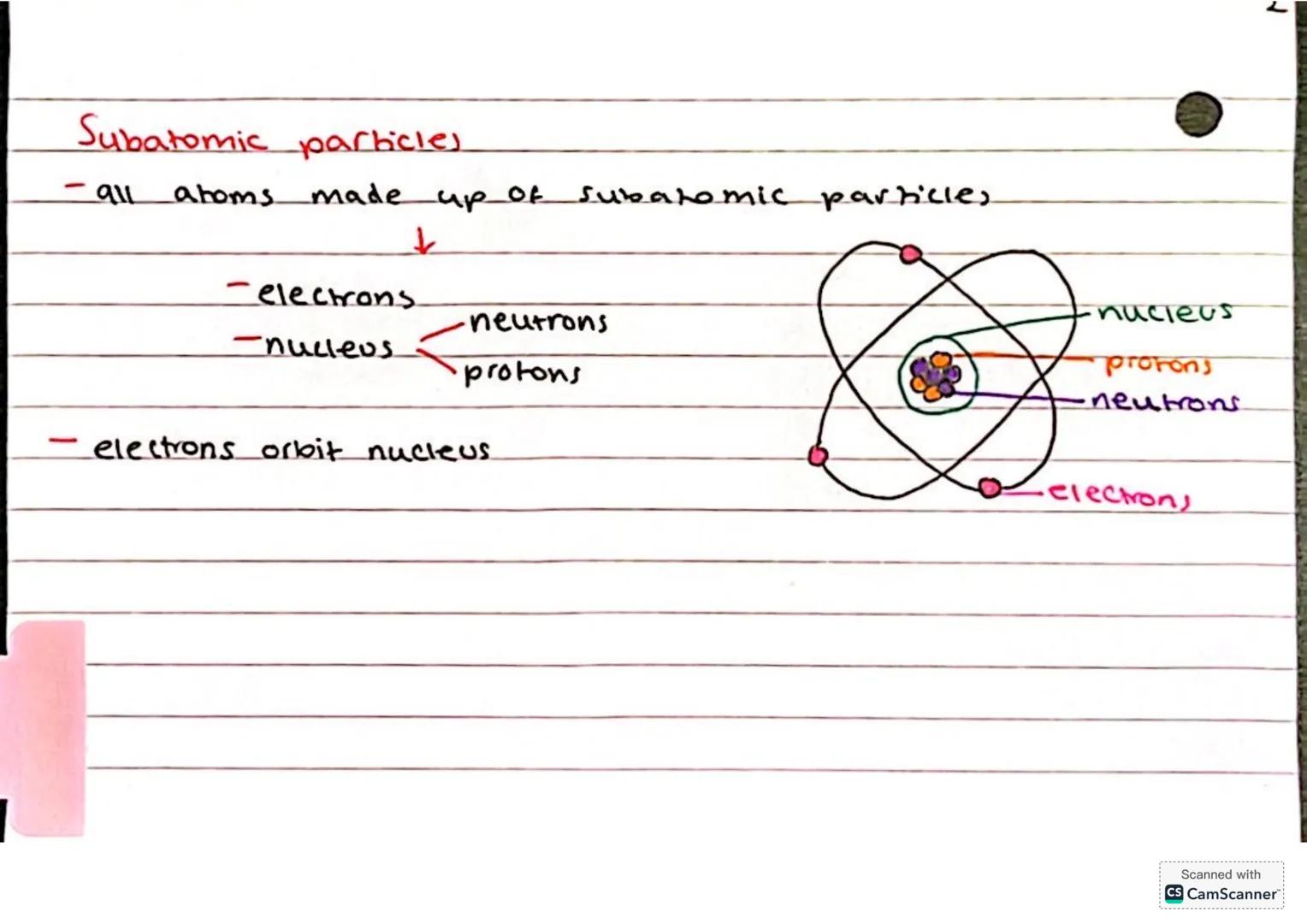
Here's where things get really interesting - atoms aren't actually the smallest particles! Every atom is made up of three types of subatomic particles that work together like a perfectly organised team.
At the centre sits the nucleus, which contains protons (positively charged) and neutrons (no charge). Racing around this nucleus are the electrons (negatively charged) in their own special orbits or shells.
Picture it like a tiny solar system - the nucleus is your sun, and electrons are planets whizzing around it. This structure explains so much about how atoms behave and bond with each other.
Remember: Protons and neutrons hang out together in the nucleus, whilst electrons orbit around the outside!

Chemical reactions are like recipes, and chemical equations are how we write them down. Every equation shows reactants (what you start with) turning into products (what you end up with), separated by an arrow.
The golden rule? Equations must be balanced because of the law of conservation of mass. This means you need the same number of each type of atom on both sides - atoms can't just disappear or appear from nowhere!
State symbols tell you what form each substance is in: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). These little letters in brackets might seem pointless, but they're actually crucial for understanding what's happening in a reaction.
Exam hack: Always check your balanced equations by counting atoms on each side - it's an easy way to pick up marks!
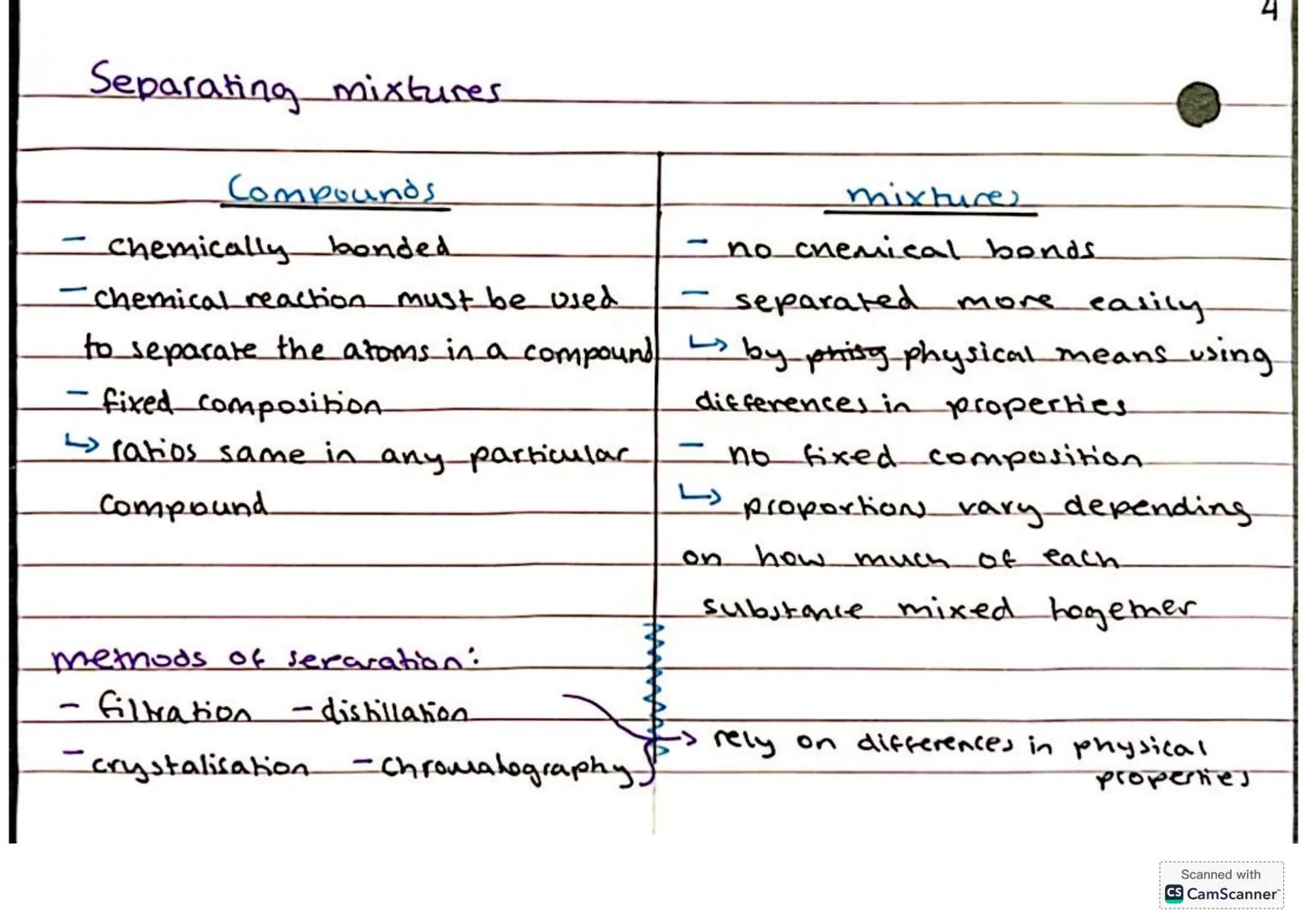
Understanding the difference between compounds and mixtures is absolutely crucial for chemistry success. Compounds form when two or more different types of atoms bond together chemically - think water (H₂O) or carbon dioxide (CO₂).
Compounds have a fixed composition and you need a chemical reaction to break them apart. Mixtures, on the other hand, are much more relaxed - they're just different substances hanging out together with no chemical bonds between them.
The brilliant thing about mixtures is that you can separate them using physical methods that rely on differences in properties. You've got filtration, distillation, crystallisation, and chromatography in your separation toolkit.
Key insight: If you can separate it easily without a chemical reaction, it's a mixture. If you need to break chemical bonds, it's a compound!
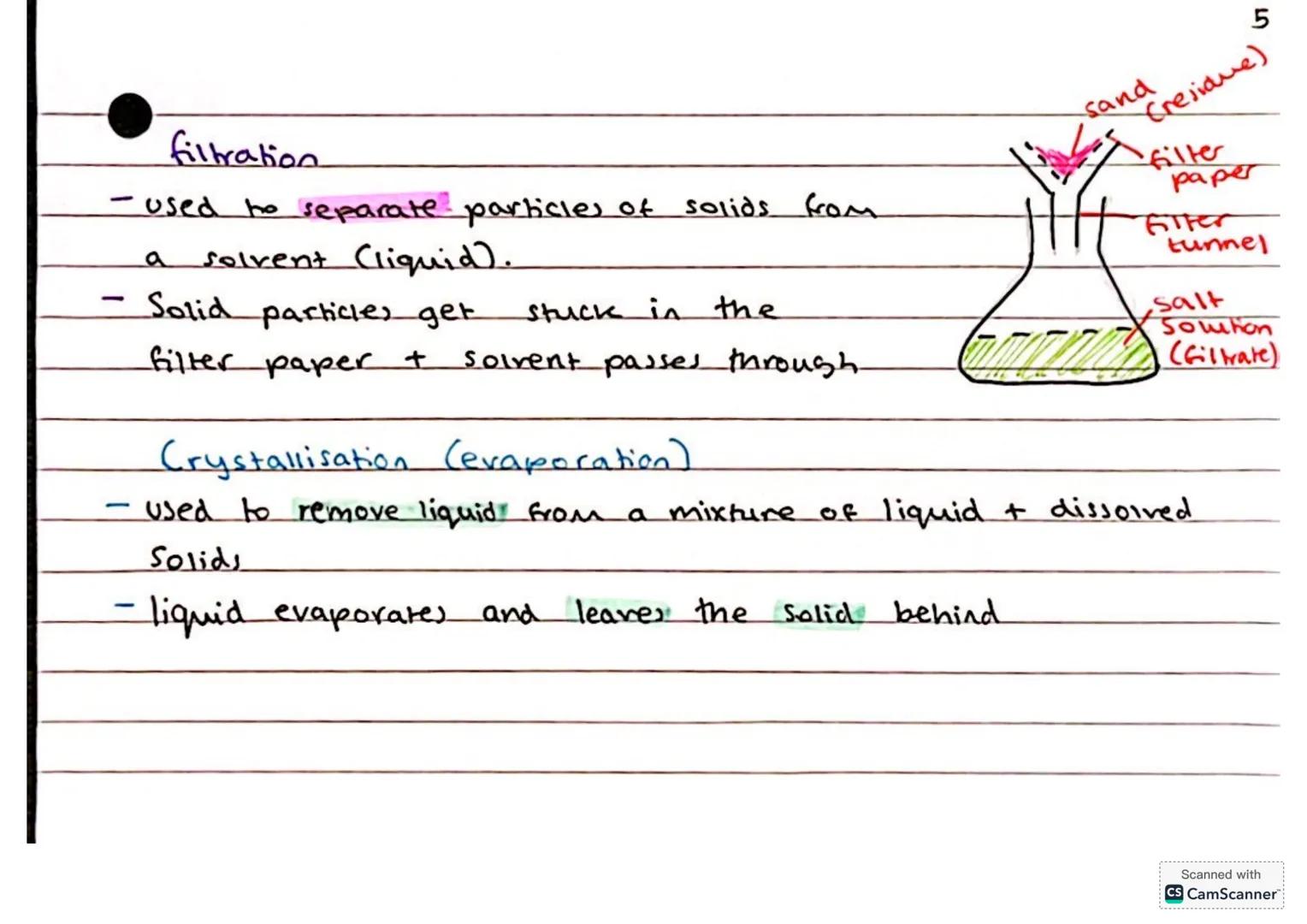
Filtration is your go-to method when you need to separate solid particles from a liquid. Think of it as a really selective bouncer - the filter paper lets the liquid through but stops the solid particles, which become the residue.
You'll use filter paper, a funnel, and a bit of patience. The liquid that passes through (called the filtrate) is now free from solid particles. It's perfect for separating sand from salt water, for example.
Crystallisation (sometimes called evaporation) works when you want to recover dissolved solids from a solution. Heat up the mixture, let the liquid evaporate, and watch as crystals of the dissolved substance appear like magic.
Pro tip: Gentle heating for crystallisation gives you better-formed crystals than rapid boiling - useful for coursework!

Distillation is like giving your mixture the VIP treatment - it separates dissolved chemicals (solutes) from the liquids they're dissolved in (solvents). This technique is absolutely essential when you need to purify liquids.
Here's how it works: heat your solution until the solvent boils and turns into vapour. This vapour travels into a condenser - essentially a cooling jacket that turns the vapour back into liquid for collection.
The clever bit is that any dissolved solids get left behind in the original flask whilst your pure liquid gets collected separately. It's like having a bouncer that only lets the liquid through!
Remember: The condenser needs cold water flowing through it constantly - no water flow means no cooling, and no cooling means no separation!

Fractional distillation takes things up a notch - it separates liquids that mix completely together (called miscible liquids) but have different boiling points. The secret weapon here is the fractionating column.
Temperature is highest at the bottom of the column and coolest at the top. Substances with higher boiling points condense lower down, whilst those with lower boiling points rise higher before condensing.
This technique is everywhere in the real world - separating crude oil into petrol, diesel, and other useful products, making alcoholic drinks like whisky and vodka, and even producing bioethanol from water.
Real-world connection: Every time you fill up a car with petrol, you're using a product of fractional distillation from crude oil refineries!

Paper chromatography is like a race where different substances compete to see how far they can travel up a piece of paper. It's brilliant for separating and identifying substances from mixtures, especially when dealing with dyes or inks.
The technique relies on different compounds dissolving differently in your chosen solvent. Dab your mixture onto chromatography paper with a capillary tube, then let the solvent soak up through the spot.
The retention factor (Rf) helps you identify substances - it's the distance the sample travelled divided by the distance the solvent travelled. Each substance has its own unique Rf value, like a chemical fingerprint.
Exam essential: Always use a pencil for your starting line in chromatography - pen ink would interfere with your results!

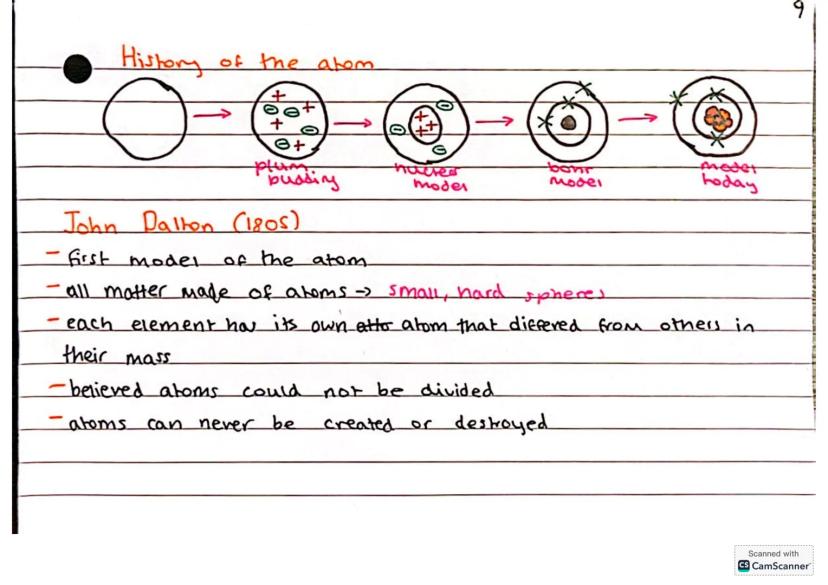
The story of atomic theory is like a scientific detective story that's still being written today. John Dalton kicked things off in 1805 with his groundbreaking ideas that all matter was made of tiny, indivisible spheres called atoms.
Dalton believed each element had its own unique type of atom that differed in mass from other elements. His key insights were that atoms couldn't be divided, created, or destroyed - pretty revolutionary thinking for the time!
This atomic model laid the foundation for modern chemistry, even though we now know atoms can actually be split and are made up of even smaller particles.
Historical context: Dalton's ideas were considered radical in 1805 - imagine trying to convince people that invisible particles made up everything around them!

JJ Thompson revolutionised atomic theory in 1897 by discovering the electron and proposing his famous "plum pudding model". He imagined atoms as clouds of positive charge with negatively charged electrons scattered throughout - like raisins in a pudding.
Ernest Rutherford completely changed the game in 1909 with his alpha scattering experiment. He fired positively charged particles at gold foil and discovered that atoms were mostly empty space with a dense, positively charged nucleus at the centre.
Rutherford's nuclear model showed that electrons orbit around this central nucleus, much like planets around the sun. This discovery revealed that atoms aren't solid spheres but are actually mostly empty space with all the mass concentrated in the tiny nucleus.
Mind-blowing fact: If an atom were the size of a football stadium, the nucleus would be smaller than a marble at the centre!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
defne
@defneguvenn
Ever wondered what makes up everything around you? Atoms are the tiny building blocks of all matter, and understanding their structure is the foundation of chemistry. From the periodic table to separating mixtures, this topic covers the essential concepts that'll... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Everything you touch, breathe, or see is made up of incredibly tiny particles called atoms. Think of them as nature's LEGO blocks - they're the smallest parts of an element that still keep all its properties.
Elements are pure substances made from just one type of atom. You'll find all known elements organised on the periodic table, each with its own symbol (like H for hydrogen or O for oxygen). The periodic table isn't random - it's arranged in columns called groups and rows called periods for good reason.
Elements in the same group have similar chemical properties, which means they behave alike in reactions. The period an element sits in tells you how many electron shells its atoms have - pretty neat, right?
Quick tip: The periodic table is like a cheat sheet for chemistry - learn to read it and you'll save loads of time in exams!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
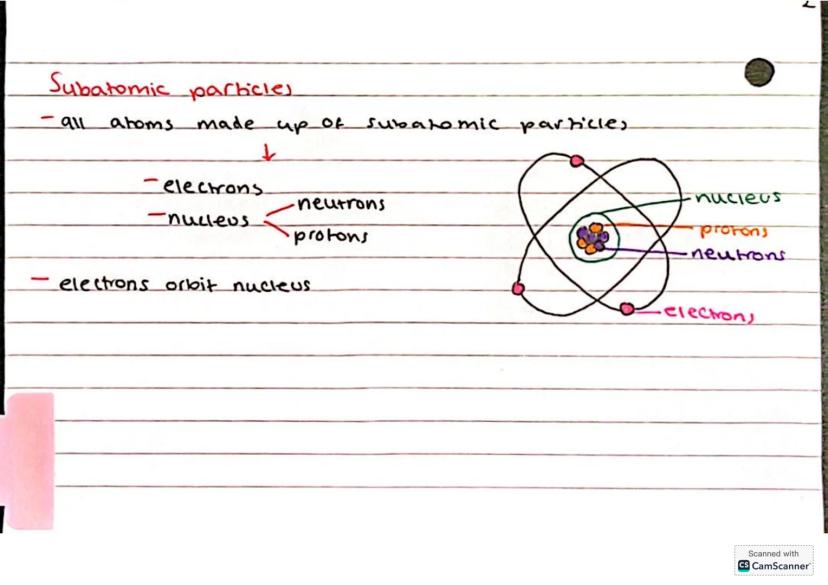
Here's where things get really interesting - atoms aren't actually the smallest particles! Every atom is made up of three types of subatomic particles that work together like a perfectly organised team.
At the centre sits the nucleus, which contains protons (positively charged) and neutrons (no charge). Racing around this nucleus are the electrons (negatively charged) in their own special orbits or shells.
Picture it like a tiny solar system - the nucleus is your sun, and electrons are planets whizzing around it. This structure explains so much about how atoms behave and bond with each other.
Remember: Protons and neutrons hang out together in the nucleus, whilst electrons orbit around the outside!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemical reactions are like recipes, and chemical equations are how we write them down. Every equation shows reactants (what you start with) turning into products (what you end up with), separated by an arrow.
The golden rule? Equations must be balanced because of the law of conservation of mass. This means you need the same number of each type of atom on both sides - atoms can't just disappear or appear from nowhere!
State symbols tell you what form each substance is in: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). These little letters in brackets might seem pointless, but they're actually crucial for understanding what's happening in a reaction.
Exam hack: Always check your balanced equations by counting atoms on each side - it's an easy way to pick up marks!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
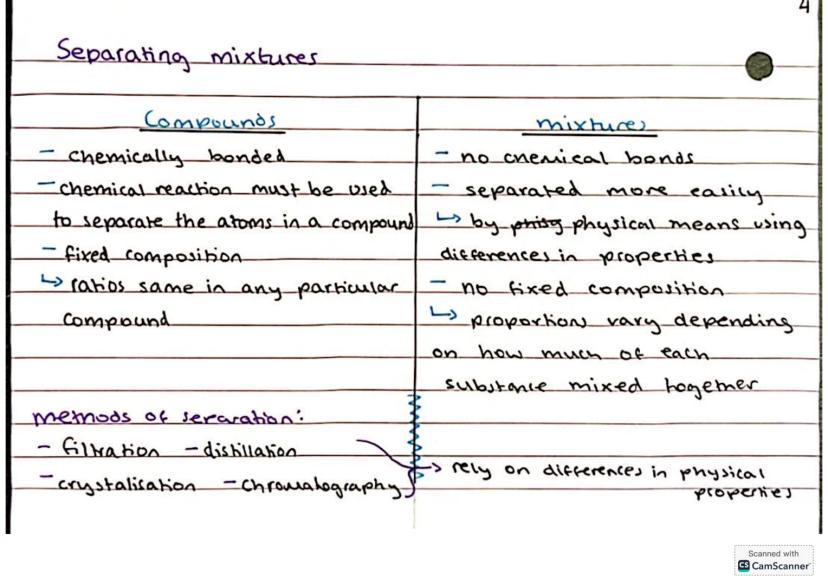
Understanding the difference between compounds and mixtures is absolutely crucial for chemistry success. Compounds form when two or more different types of atoms bond together chemically - think water (H₂O) or carbon dioxide (CO₂).
Compounds have a fixed composition and you need a chemical reaction to break them apart. Mixtures, on the other hand, are much more relaxed - they're just different substances hanging out together with no chemical bonds between them.
The brilliant thing about mixtures is that you can separate them using physical methods that rely on differences in properties. You've got filtration, distillation, crystallisation, and chromatography in your separation toolkit.
Key insight: If you can separate it easily without a chemical reaction, it's a mixture. If you need to break chemical bonds, it's a compound!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
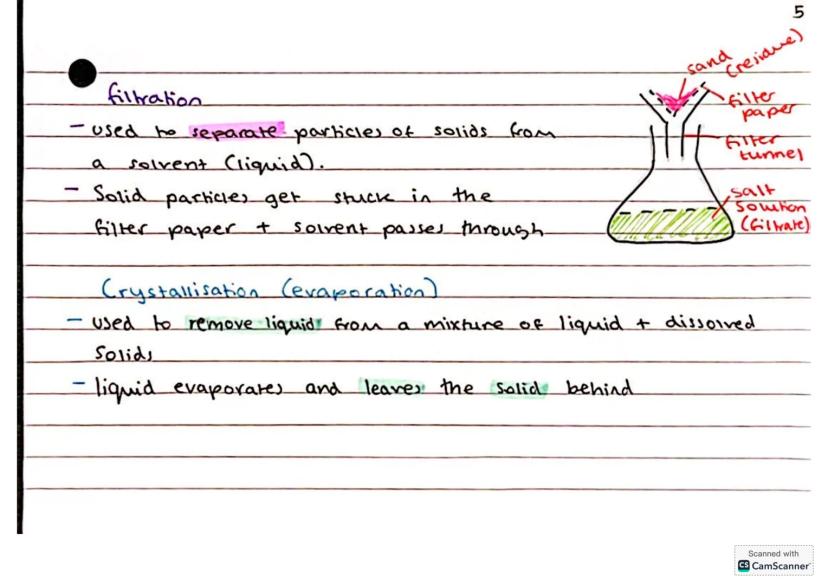
Filtration is your go-to method when you need to separate solid particles from a liquid. Think of it as a really selective bouncer - the filter paper lets the liquid through but stops the solid particles, which become the residue.
You'll use filter paper, a funnel, and a bit of patience. The liquid that passes through (called the filtrate) is now free from solid particles. It's perfect for separating sand from salt water, for example.
Crystallisation (sometimes called evaporation) works when you want to recover dissolved solids from a solution. Heat up the mixture, let the liquid evaporate, and watch as crystals of the dissolved substance appear like magic.
Pro tip: Gentle heating for crystallisation gives you better-formed crystals than rapid boiling - useful for coursework!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Distillation is like giving your mixture the VIP treatment - it separates dissolved chemicals (solutes) from the liquids they're dissolved in (solvents). This technique is absolutely essential when you need to purify liquids.
Here's how it works: heat your solution until the solvent boils and turns into vapour. This vapour travels into a condenser - essentially a cooling jacket that turns the vapour back into liquid for collection.
The clever bit is that any dissolved solids get left behind in the original flask whilst your pure liquid gets collected separately. It's like having a bouncer that only lets the liquid through!
Remember: The condenser needs cold water flowing through it constantly - no water flow means no cooling, and no cooling means no separation!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
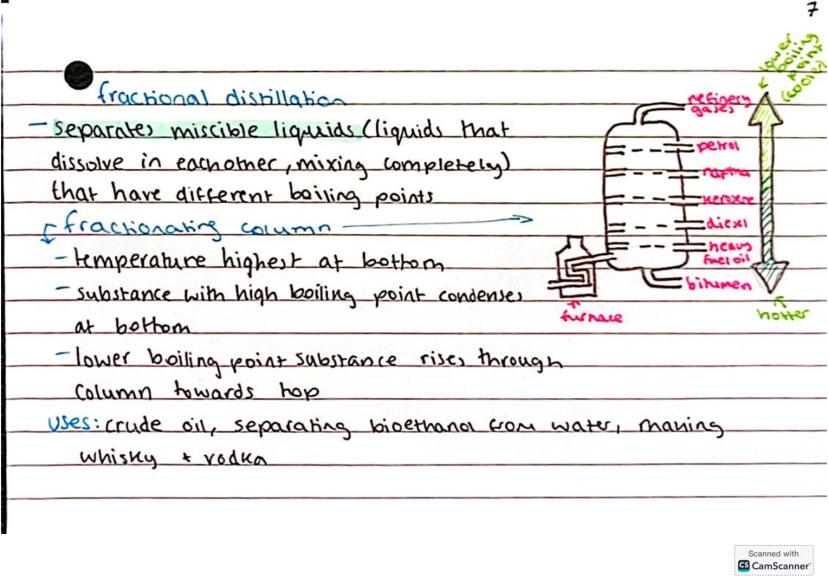
Fractional distillation takes things up a notch - it separates liquids that mix completely together (called miscible liquids) but have different boiling points. The secret weapon here is the fractionating column.
Temperature is highest at the bottom of the column and coolest at the top. Substances with higher boiling points condense lower down, whilst those with lower boiling points rise higher before condensing.
This technique is everywhere in the real world - separating crude oil into petrol, diesel, and other useful products, making alcoholic drinks like whisky and vodka, and even producing bioethanol from water.
Real-world connection: Every time you fill up a car with petrol, you're using a product of fractional distillation from crude oil refineries!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Paper chromatography is like a race where different substances compete to see how far they can travel up a piece of paper. It's brilliant for separating and identifying substances from mixtures, especially when dealing with dyes or inks.
The technique relies on different compounds dissolving differently in your chosen solvent. Dab your mixture onto chromatography paper with a capillary tube, then let the solvent soak up through the spot.
The retention factor (Rf) helps you identify substances - it's the distance the sample travelled divided by the distance the solvent travelled. Each substance has its own unique Rf value, like a chemical fingerprint.
Exam essential: Always use a pencil for your starting line in chromatography - pen ink would interfere with your results!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The story of atomic theory is like a scientific detective story that's still being written today. John Dalton kicked things off in 1805 with his groundbreaking ideas that all matter was made of tiny, indivisible spheres called atoms.
Dalton believed each element had its own unique type of atom that differed in mass from other elements. His key insights were that atoms couldn't be divided, created, or destroyed - pretty revolutionary thinking for the time!
This atomic model laid the foundation for modern chemistry, even though we now know atoms can actually be split and are made up of even smaller particles.
Historical context: Dalton's ideas were considered radical in 1805 - imagine trying to convince people that invisible particles made up everything around them!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
JJ Thompson revolutionised atomic theory in 1897 by discovering the electron and proposing his famous "plum pudding model". He imagined atoms as clouds of positive charge with negatively charged electrons scattered throughout - like raisins in a pudding.
Ernest Rutherford completely changed the game in 1909 with his alpha scattering experiment. He fired positively charged particles at gold foil and discovered that atoms were mostly empty space with a dense, positively charged nucleus at the centre.
Rutherford's nuclear model showed that electrons orbit around this central nucleus, much like planets around the sun. This discovery revealed that atoms aren't solid spheres but are actually mostly empty space with all the mass concentrated in the tiny nucleus.
Mind-blowing fact: If an atom were the size of a football stadium, the nucleus would be smaller than a marble at the centre!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
4
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user