Atoms and Basic Chemistry Concepts
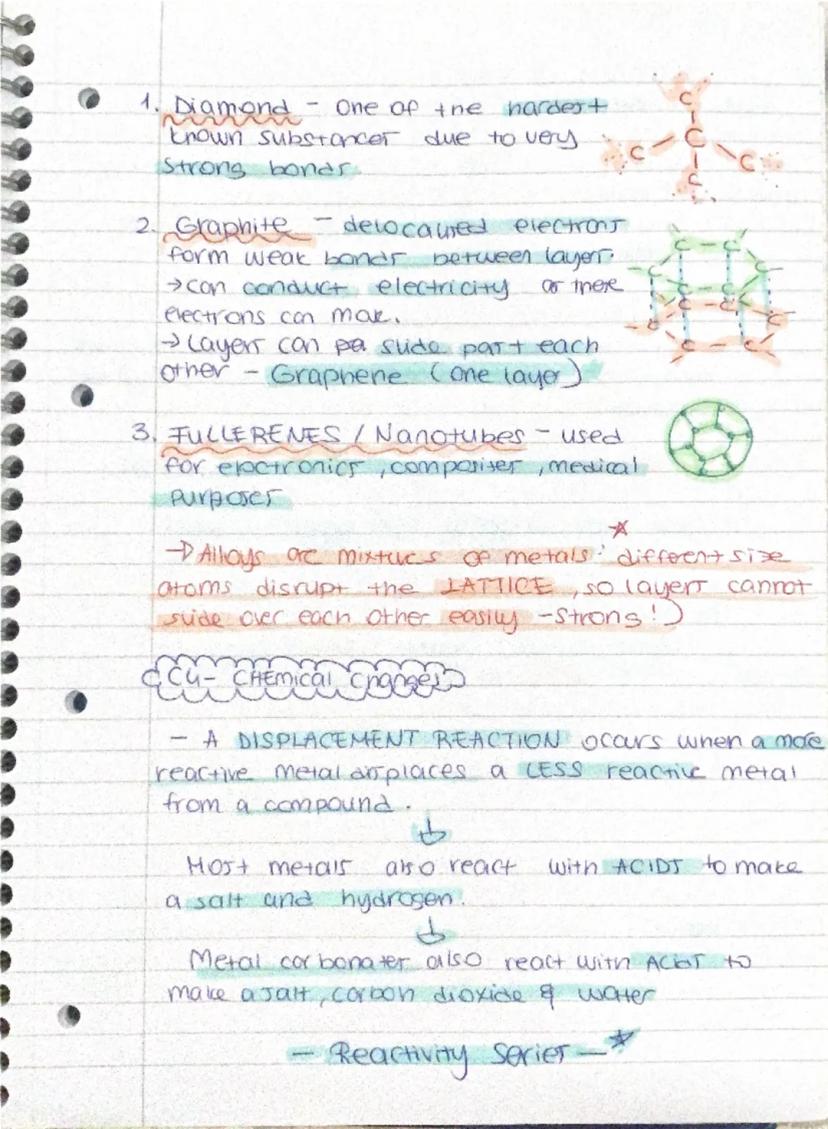
Everything you touch is made of atoms - they're literally the building blocks of the universe. An element contains only one type of atom (like pure oxygen or magnesium), whilst a compound has different atoms chemically bonded together (like water, which is hydrogen and oxygen stuck together).
Mixtures are completely different because the substances aren't chemically bonded - think of air, which is just different gases floating about together. You can separate mixtures using various techniques, but compounds need chemical reactions to break apart.
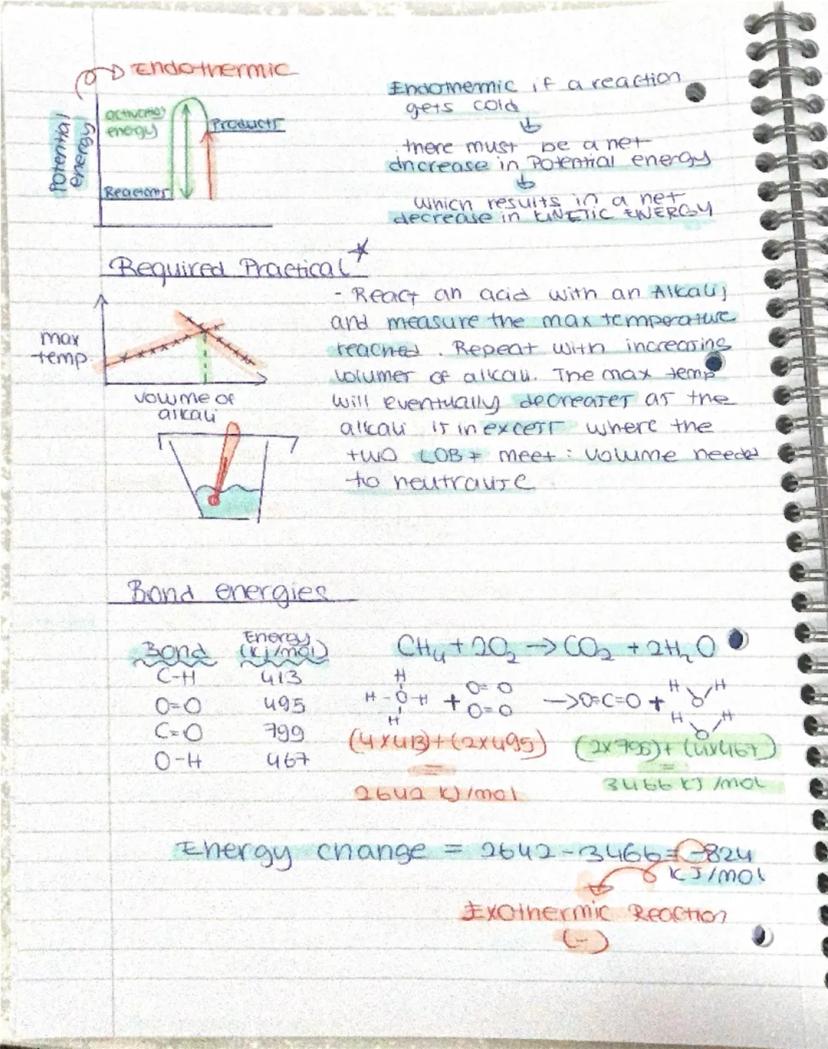
Matter exists in three main states - solid, liquid, and gas. In solids, particles vibrate in fixed positions like people in assigned cinema seats. Liquids let particles move past each other freely, whilst gases have particles zooming around randomly at high speeds with loads of space between them.
Remember: You need energy (heat) to overcome the forces holding particles together when melting or evaporating substances!
The history of atomic structure is like a detective story. John Dalton started it all, then JJ Thompson proposed the "plum pudding model." Ernest Rutherford discovered the tiny, positively charged nucleus by firing particles at gold foil, and Niels Bohr worked out that electrons exist in energy levels around the nucleus.