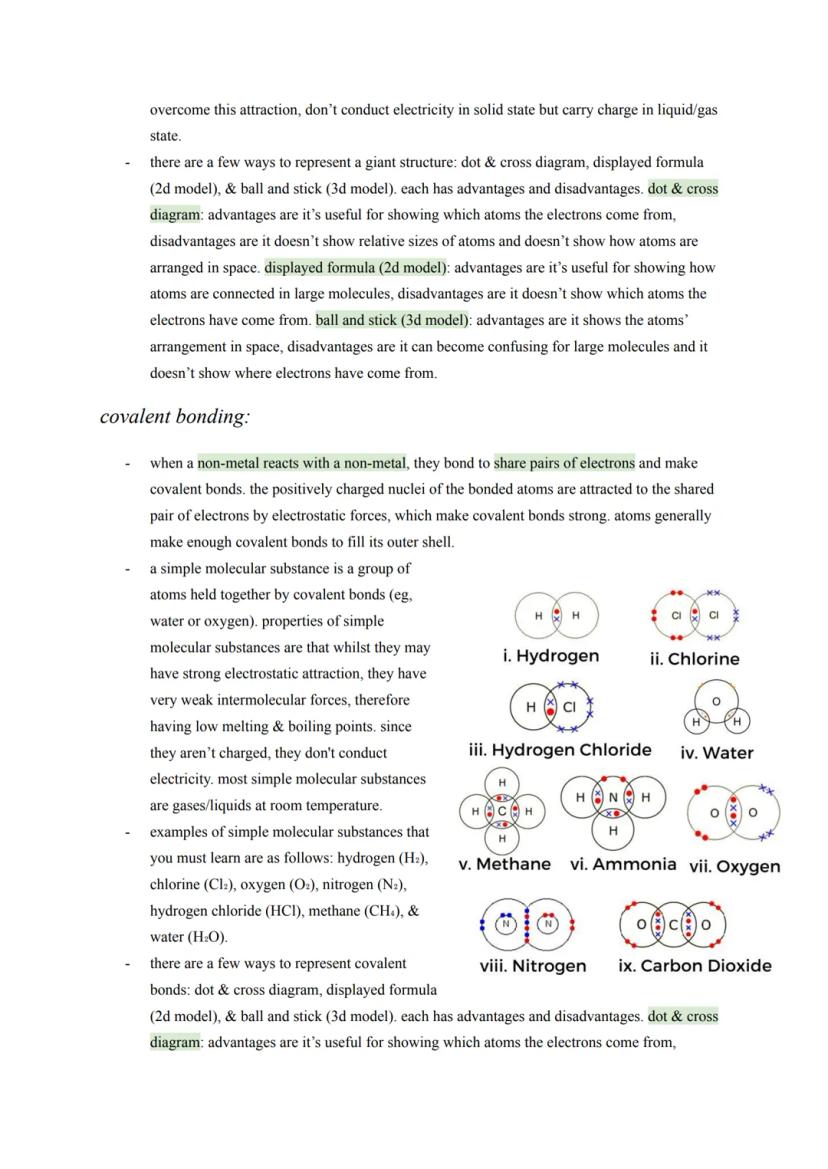
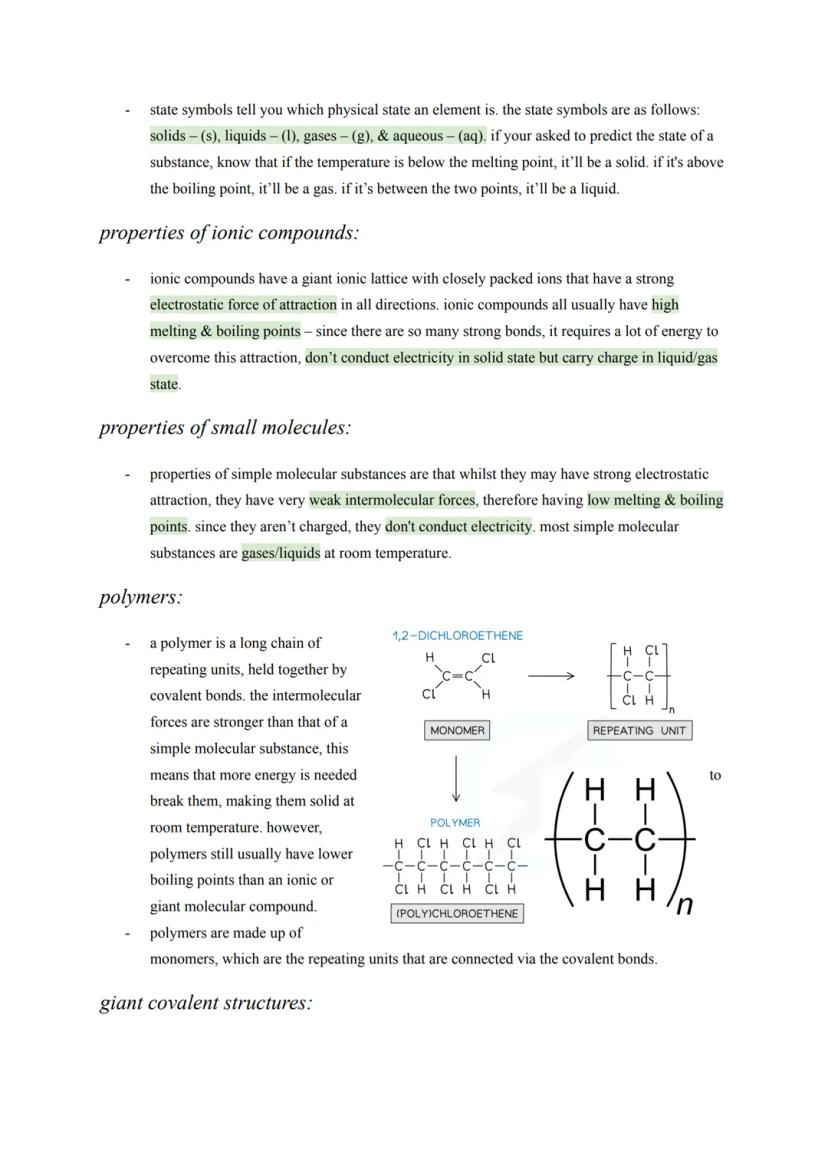
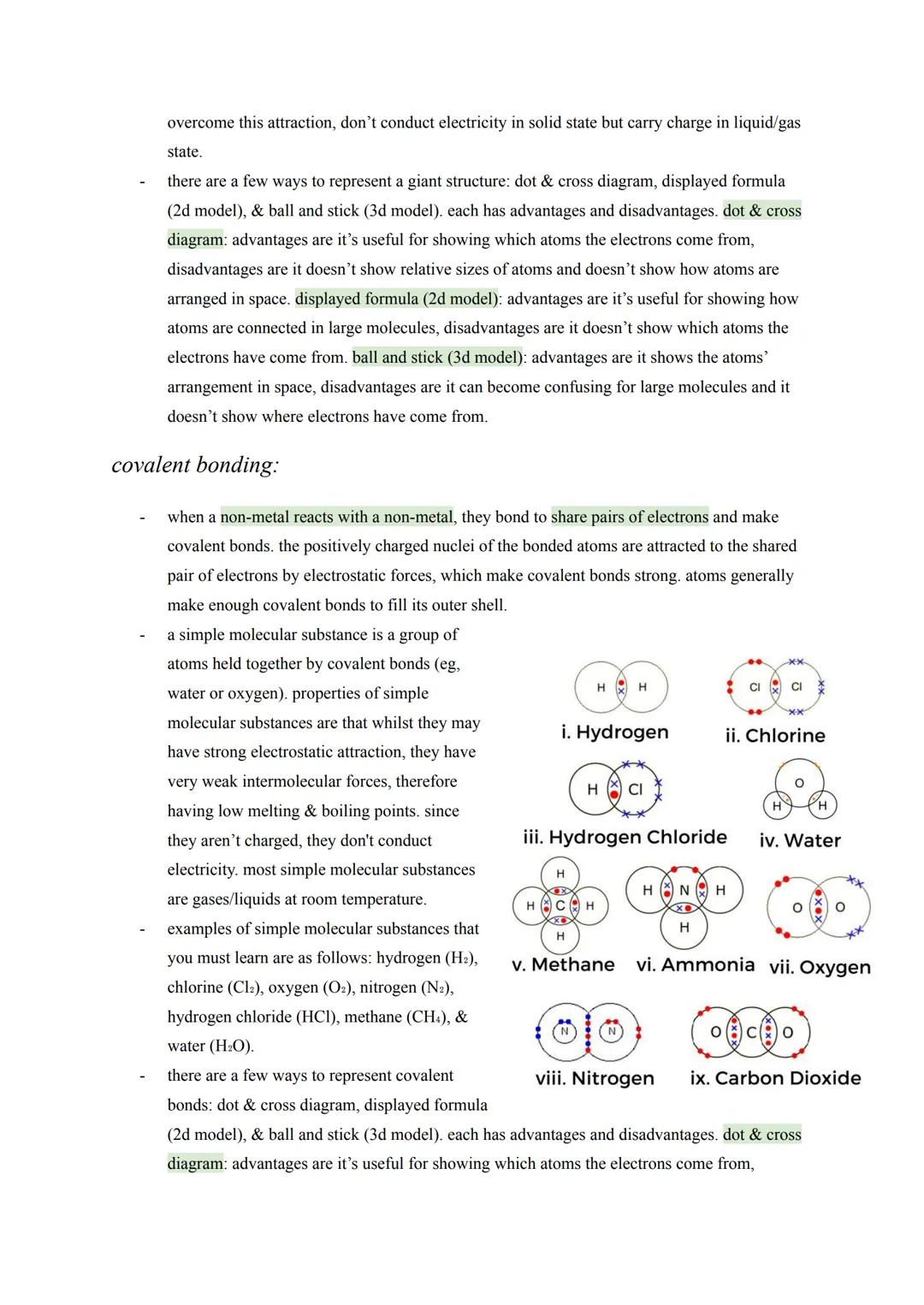
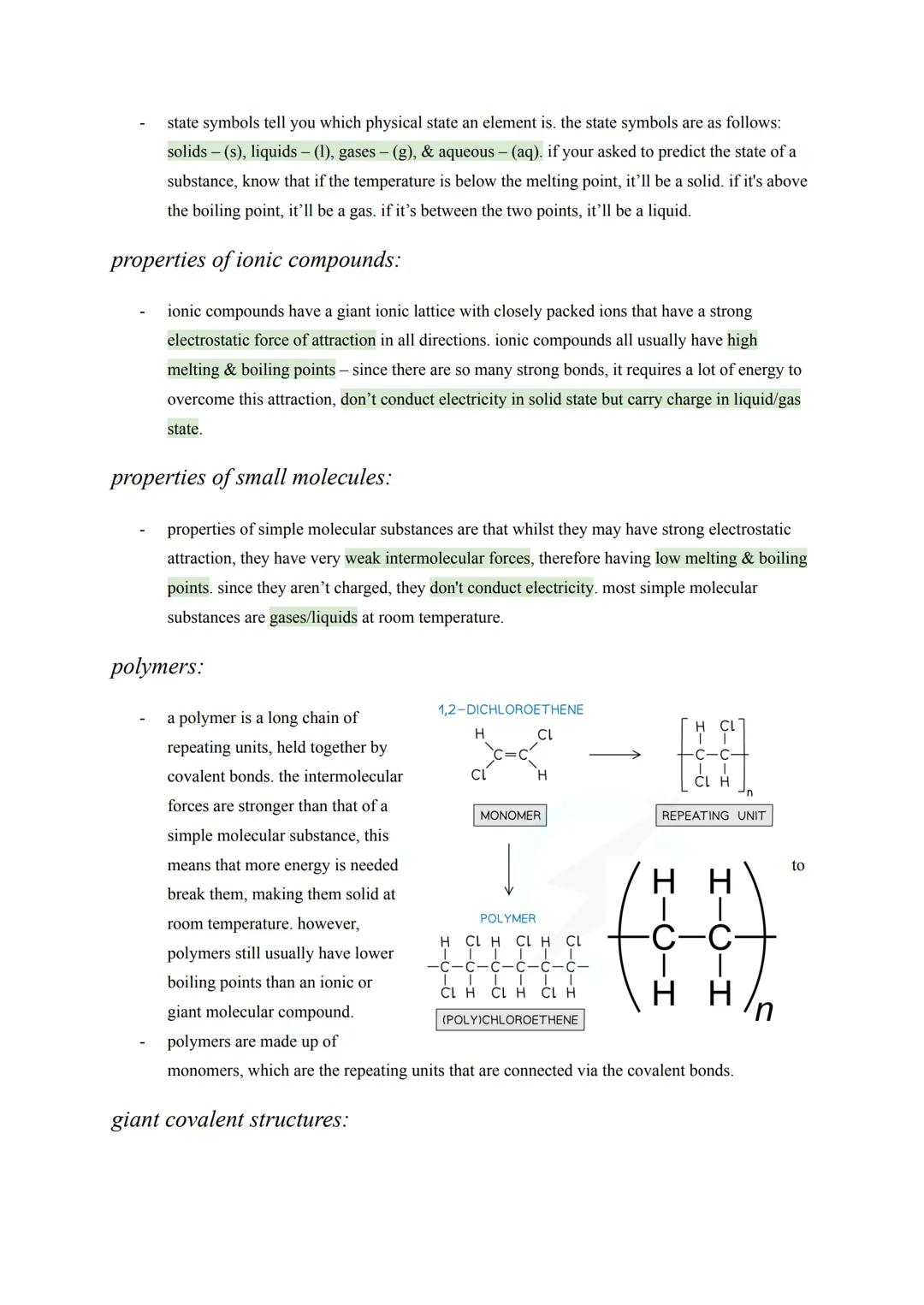
Advanced Carbon Structures
Graphene takes graphite's concept to the extreme - it's essentially a single layer of graphite that's one atom thick. This creates an incredibly strong yet lightweight 2D material with excellent electrical conductivity thanks to delocalised electrons.
Fullerenes represent carbon's most creative side, forming closed cages, tubes, or hollow spheres. Buckminsterfullerene was the first discovered - a football-shaped molecule with 60 carbon atoms arranged in hexagons and pentagons.
These structures have exciting applications: fullerenes can deliver drugs by acting as molecular cages, their large surface areas make them brilliant industrial catalysts, and they form nanotubes - tiny carbon cylinders with incredible strength and conductivity. Nanotubes are revolutionising electronics and creating super-strong, lightweight composite materials.
Future materials: Fullerenes and nanotubes represent cutting-edge nanotechnology - their unique properties are opening up possibilities from targeted medical treatments to space-age materials.