Ever wondered how chemists know exactly how much of each... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
73
•
6 Feb 2026
•
G
@gurneet
Ever wondered how chemists know exactly how much of each... Show more









Here's the golden rule of chemistry: atoms never disappear or magically appear during reactions. The law of conservation of mass means whatever goes in must come out - just rearranged differently.
This is why we can balance chemical equations. Think of it like a recipe where you need equal amounts on both sides. The relative formula mass (Mr) is simply adding up all the atomic masses in a compound's formula.
Mass changes during reactions usually happen when gases escape or get absorbed. If your metal gets heavier when heated, it's probably reacting with oxygen from the air. If your compound gets lighter, a gas like CO₂ has likely escaped.
Quick Tip: If mass seems to change, look for gases! They're often the sneaky culprit.

Think of a mole as chemistry's way of counting - like saying "a dozen" but for atoms and molecules. One mole always contains 6.02 × 10²³ particles (that's Avogadro's constant), whether you're counting atoms, molecules, or ions.
The brilliant thing about moles is that one mole of any substance has a mass in grams equal to its Mr. So one mole of carbon weighs exactly 12g.
Chemical equations become much clearer with moles. When you see Mg + 2HCl → MgCl₂ + H₂, it's telling you exactly how many moles of each substance react together - like a perfectly balanced recipe.
Remember: Moles = Mass ÷ Mr. This simple formula will be your best friend!

Once you've got the hang of moles, calculating masses becomes straightforward. It's a three-step process: convert mass to moles, use the equation's ratio, then convert back to mass.
Let's say you have 6g of magnesium and want to know how much hydrogen gas you'll get. First, find moles of Mg . The equation shows 1:1 ratio, so you'll get 0.25 mol of H₂. Finally, convert to mass .
Balancing equations using masses works backwards - you calculate moles from given masses, find the simplest ratio, then write the balanced equation. It's like reverse-engineering a recipe from the ingredients you used.
Pro Tip: Always check your mole ratios match the balanced equation - it's your safety net!

Balancing equations from experimental data is actually quite logical. You calculate moles of each substance, find the simplest whole number ratio, then write your balanced equation using those ratios.
Limiting reactants are like the ingredient that runs out first when cooking. Even if you have loads of other reactants, the reaction stops when the limiting reactant is used up. This determines how much product you can actually make.
To find the limiting reactant, calculate how much of each reactant you'd need based on the equation's ratios. Whichever one you don't have enough of is your limiting reactant - and it controls your final yield.
Think of it like baking: If a cake needs 2 eggs per cup of flour, but you only have 1 egg and 3 cups of flour, the eggs limit how much cake you can make!

Concentration tells you how much stuff is dissolved in your solution. It's measured in g/dm³ - grams of solute per cubic decimetre of solution. More solute or less solvent means higher concentration.
The formula is simple: Concentration = Mass of solute ÷ Volume of solution. Remember to convert cm³ to dm³ by dividing by 1000 - this trips up loads of students in exams!
Limiting reactants in solutions work the same way. Calculate moles of each reactant, work out which one runs out first using the equation's ratios, then use that to find your maximum product yield.
Units matter: Always double-check you're using dm³, not cm³, or your answer will be 1000 times too big!

Real reactions rarely give you exactly what the maths predicts. Percentage yield compares what you actually got to what you should have got theoretically. It's calculated as: (Actual yield ÷ Theoretical yield) × 100.
Why don't you get 100% yield? Reactions might be reversible, some product gets lost during separation, or side reactions happen. It's frustrating but totally normal!
Atom economy measures how much of your starting materials end up as useful product rather than waste. High atom economy means less waste, which is better for the environment and cheaper for companies.
Industry tip: Companies love reactions with high atom economy and high percentage yield - more profit, less waste!

Calculating theoretical yield follows the same pattern: balanced equation, find moles of reactant, use mole ratio, convert to mass of product. This gives you the maximum possible amount.
For industry, multiple factors matter: atom economy (less waste), percentage yield (more product), reaction rate , and whether by-products are useful. It's not just about the chemistry - it's about the money too!
The best industrial processes tick all the boxes - high yield, high atom economy, fast reactions, and valuable by-products. That's why developing new reactions is so important for sustainable chemistry.
Real world: Even a reaction with 80% yield might be preferred if it's much faster or produces valuable by-products!

Concentration in mol/dm³ is often more useful than g/dm³ because it directly tells you how many particles you have. Calculate it using: moles of solute ÷ volume in dm³.
You can work out unknown concentrations if you know the volumes and one concentration from a reaction. It's all about using the mole ratios from your balanced equation.
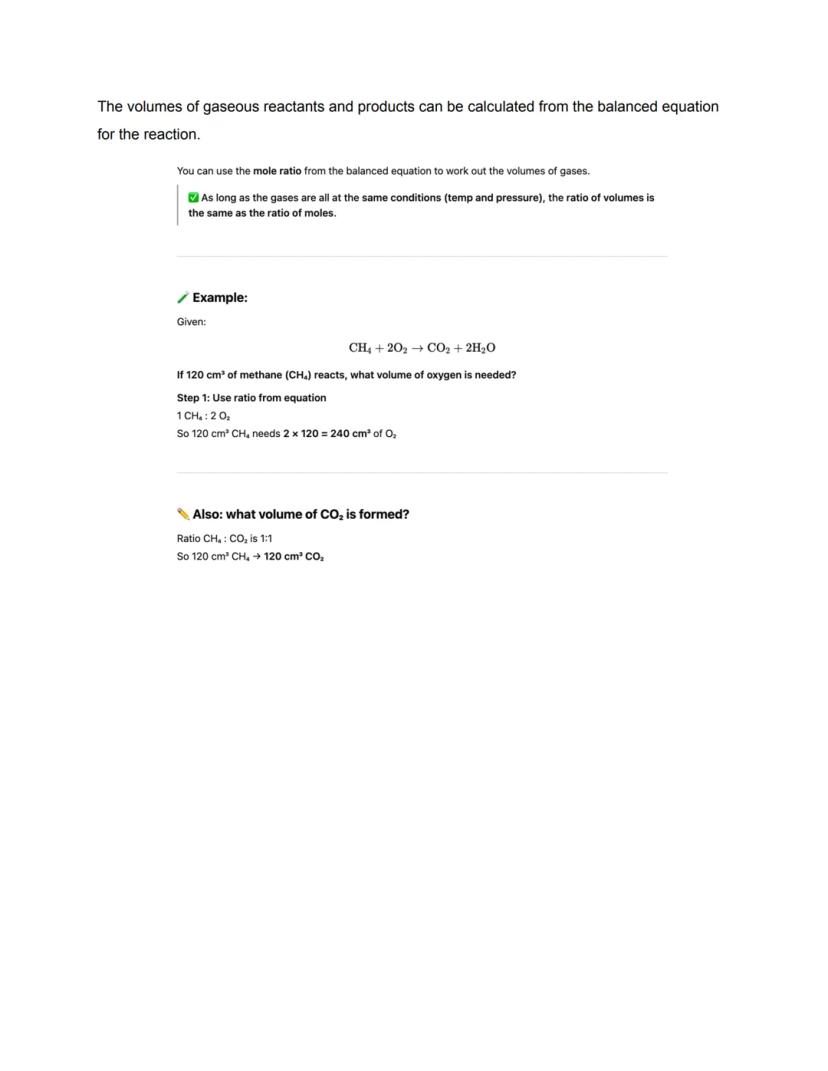
Gas volumes follow a brilliant rule: equal moles occupy equal volumes under the same conditions. At room temperature and pressure, one mole of any gas occupies 24 dm³. This makes calculations much easier!
Gas shortcut: Volume = moles × 24 dm³ at RTP. No need to worry about different gases having different volumes!
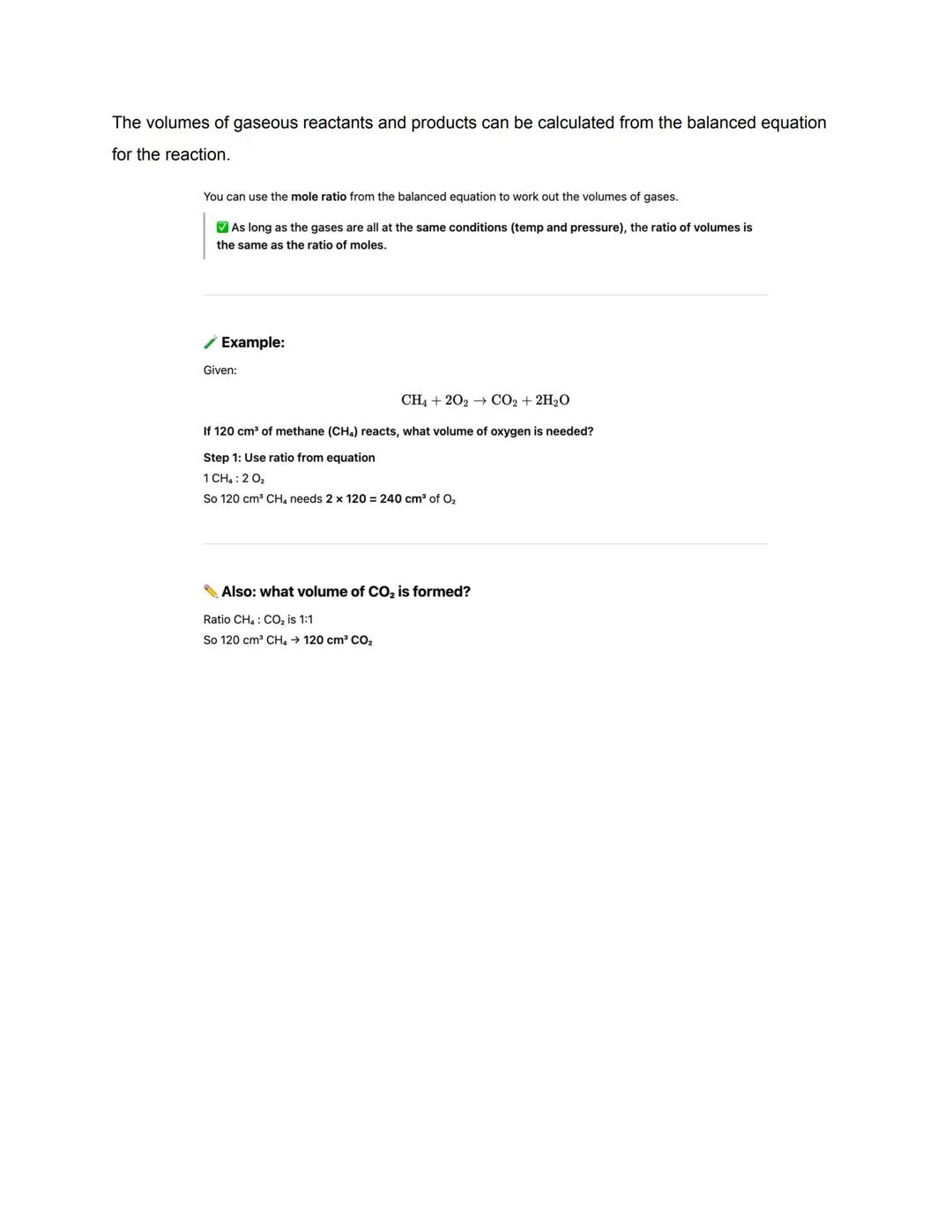
Calculating gas volumes from equations is surprisingly straightforward. Since equal moles of gases occupy equal volumes under the same conditions, the volume ratios match the mole ratios from your balanced equation.
If your equation shows CH₄ + 2O₂ → CO₂ + 2H₂O, then 1 volume of methane needs 2 volumes of oxygen and produces 1 volume of carbon dioxide. No complex calculations needed - just use the ratios directly!
This only works when all gases are at the same temperature and pressure. If conditions change, you'd need to account for that, but exam questions usually keep things simple.
Volume magic: The coefficients in your balanced equation directly tell you the volume ratios - chemistry made easy!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
G
@gurneet
Ever wondered how chemists know exactly how much of each substance they need for a reaction, or why some experiments don't give you as much product as expected? Quantitative chemistry is all about the maths behind chemical reactions - from... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's the golden rule of chemistry: atoms never disappear or magically appear during reactions. The law of conservation of mass means whatever goes in must come out - just rearranged differently.
This is why we can balance chemical equations. Think of it like a recipe where you need equal amounts on both sides. The relative formula mass (Mr) is simply adding up all the atomic masses in a compound's formula.
Mass changes during reactions usually happen when gases escape or get absorbed. If your metal gets heavier when heated, it's probably reacting with oxygen from the air. If your compound gets lighter, a gas like CO₂ has likely escaped.
Quick Tip: If mass seems to change, look for gases! They're often the sneaky culprit.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of a mole as chemistry's way of counting - like saying "a dozen" but for atoms and molecules. One mole always contains 6.02 × 10²³ particles (that's Avogadro's constant), whether you're counting atoms, molecules, or ions.
The brilliant thing about moles is that one mole of any substance has a mass in grams equal to its Mr. So one mole of carbon weighs exactly 12g.
Chemical equations become much clearer with moles. When you see Mg + 2HCl → MgCl₂ + H₂, it's telling you exactly how many moles of each substance react together - like a perfectly balanced recipe.
Remember: Moles = Mass ÷ Mr. This simple formula will be your best friend!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Once you've got the hang of moles, calculating masses becomes straightforward. It's a three-step process: convert mass to moles, use the equation's ratio, then convert back to mass.
Let's say you have 6g of magnesium and want to know how much hydrogen gas you'll get. First, find moles of Mg . The equation shows 1:1 ratio, so you'll get 0.25 mol of H₂. Finally, convert to mass .
Balancing equations using masses works backwards - you calculate moles from given masses, find the simplest ratio, then write the balanced equation. It's like reverse-engineering a recipe from the ingredients you used.
Pro Tip: Always check your mole ratios match the balanced equation - it's your safety net!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Balancing equations from experimental data is actually quite logical. You calculate moles of each substance, find the simplest whole number ratio, then write your balanced equation using those ratios.
Limiting reactants are like the ingredient that runs out first when cooking. Even if you have loads of other reactants, the reaction stops when the limiting reactant is used up. This determines how much product you can actually make.
To find the limiting reactant, calculate how much of each reactant you'd need based on the equation's ratios. Whichever one you don't have enough of is your limiting reactant - and it controls your final yield.
Think of it like baking: If a cake needs 2 eggs per cup of flour, but you only have 1 egg and 3 cups of flour, the eggs limit how much cake you can make!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Concentration tells you how much stuff is dissolved in your solution. It's measured in g/dm³ - grams of solute per cubic decimetre of solution. More solute or less solvent means higher concentration.
The formula is simple: Concentration = Mass of solute ÷ Volume of solution. Remember to convert cm³ to dm³ by dividing by 1000 - this trips up loads of students in exams!
Limiting reactants in solutions work the same way. Calculate moles of each reactant, work out which one runs out first using the equation's ratios, then use that to find your maximum product yield.
Units matter: Always double-check you're using dm³, not cm³, or your answer will be 1000 times too big!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Real reactions rarely give you exactly what the maths predicts. Percentage yield compares what you actually got to what you should have got theoretically. It's calculated as: (Actual yield ÷ Theoretical yield) × 100.
Why don't you get 100% yield? Reactions might be reversible, some product gets lost during separation, or side reactions happen. It's frustrating but totally normal!
Atom economy measures how much of your starting materials end up as useful product rather than waste. High atom economy means less waste, which is better for the environment and cheaper for companies.
Industry tip: Companies love reactions with high atom economy and high percentage yield - more profit, less waste!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Calculating theoretical yield follows the same pattern: balanced equation, find moles of reactant, use mole ratio, convert to mass of product. This gives you the maximum possible amount.
For industry, multiple factors matter: atom economy (less waste), percentage yield (more product), reaction rate , and whether by-products are useful. It's not just about the chemistry - it's about the money too!
The best industrial processes tick all the boxes - high yield, high atom economy, fast reactions, and valuable by-products. That's why developing new reactions is so important for sustainable chemistry.
Real world: Even a reaction with 80% yield might be preferred if it's much faster or produces valuable by-products!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Concentration in mol/dm³ is often more useful than g/dm³ because it directly tells you how many particles you have. Calculate it using: moles of solute ÷ volume in dm³.
You can work out unknown concentrations if you know the volumes and one concentration from a reaction. It's all about using the mole ratios from your balanced equation.
Gas volumes follow a brilliant rule: equal moles occupy equal volumes under the same conditions. At room temperature and pressure, one mole of any gas occupies 24 dm³. This makes calculations much easier!
Gas shortcut: Volume = moles × 24 dm³ at RTP. No need to worry about different gases having different volumes!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Calculating gas volumes from equations is surprisingly straightforward. Since equal moles of gases occupy equal volumes under the same conditions, the volume ratios match the mole ratios from your balanced equation.
If your equation shows CH₄ + 2O₂ → CO₂ + 2H₂O, then 1 volume of methane needs 2 volumes of oxygen and produces 1 volume of carbon dioxide. No complex calculations needed - just use the ratios directly!
This only works when all gases are at the same temperature and pressure. If conditions change, you'd need to account for that, but exam questions usually keep things simple.
Volume magic: The coefficients in your balanced equation directly tell you the volume ratios - chemistry made easy!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
2
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user