Chemistry practicals are essential for your GCSE course, giving you... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
110
•
8 Feb 2026
•
Nema x friday
@nemaxfriday
Chemistry practicals are essential for your GCSE course, giving you... Show more









Ever wondered how to make those brilliant blue crystals you see in chemistry labs? This practical shows you exactly how to prepare copper sulfate from copper oxide - and it's surprisingly straightforward once you know the steps.
You start with a fixed volume of sulfuric acid as your limiting reactant, then heat it until boiling. The clever bit is adding copper oxide bit by bit whilst stirring - you'll know it's working when the solution turns that gorgeous blue colour. Keep adding copper oxide until some powder remains unreacted, which tells you all the acid has been used up.
The final steps involve filtration to remove excess copper oxide, then gentle heating in an evaporating basin until half the solution remains. Leave it for 24 hours and you'll have proper copper sulfate crystals to scrape up and dry.
Top Tip: Always add the copper oxide gradually - if you dump it all in at once, the reaction might be too vigorous and you could miss the endpoint!

Titration is your go-to method for finding unknown concentrations, and this practical uses it to work out exactly how much acid neutralises a known amount of alkali. You'll be using this technique loads in A-levels, so getting comfortable with it now is brilliant.
Start by using a pipette to measure exactly 25 cm³ of sodium hydroxide into a conical flask - the conical shape reduces splashing risk. Add a few drops of phenolphthalein indicator, which stays pink in alkaline solutions but turns colourless when neutral.
Fill your burette with the unknown acid and slowly add it whilst swirling the flask. Once you see the colour starting to change, go drop by drop until you hit the end point - that magic moment when it goes from pink to completely clear. Always read the burette at eye level, using the bottom of the meniscus for accuracy.
Remember: The first titration is just a rough run - you'll need to repeat it several times to get consistent results for your calculations.

Electrolysis might seem complex, but this practical breaks it down into manageable chunks using two different solutions. You're essentially using electricity to split compounds apart, and different ions behave in predictable ways.
For copper chloride solution, set up your carbon electrodes in a petri dish with holes, making sure they don't touch (short circuits are bad news!). At 4V, you'll see brown copper forming at the cathode (negative electrode) because copper is less reactive than hydrogen. The anode produces chlorine gas - you can test this with blue litmus paper, which gets bleached.
Sodium chloride gives different results because sodium is more reactive than hydrogen. This means hydrogen gas bubbles off at the cathode instead of sodium metal, whilst chlorine still forms at the anode. You can test the hydrogen with a lit splint - it makes that classic squeaky pop sound.
Safety Note: Always work in a well-ventilated area when producing chlorine gas, and never let the electrodes touch each other!

This practical demonstrates exothermic reactions perfectly, showing how neutralisation between hydrochloric acid and sodium hydroxide releases energy as heat. You'll see exactly how changing reactant amounts affects temperature rise.
Use a polystyrene cup inside a beaker for insulation, starting with 30 cm³ of hydrochloric acid. Measure its temperature, then add 5 cm³ of sodium hydroxide solution through a plastic lid with your thermometer poking through. Stir gently and record the maximum temperature reached.
Repeat with increasing volumes of sodium hydroxide (10 cm³, 15 cm³, etc.) and you'll notice the temperature rises as more reactants are available. However, there's a twist - beyond a certain point, the temperature actually starts dropping again.
This happens because you've got excess sodium hydroxide that can't react, plus the energy released gets spread through a larger volume of solution. It's a brilliant example of how limiting reactants work in practice.
Key Point: The polystyrene cup and lid aren't just random equipment choices - they're essential for reducing heat loss and getting accurate results.

Rate of reaction practicals are fantastic for understanding how concentration affects how fast chemical changes happen. This one uses the classic disappearing cross method with sodium thiosulfate and hydrochloric acid.
The reaction produces sulfur as a cloudy precipitate, so you time how long it takes for a black cross underneath your flask to disappear. Start with 10 cm³ of each reactant, mix them quickly, and start your stopwatch immediately. When you can no longer see the cross, record the time and temperature.
Repeat with different concentrations of sodium thiosulfate - you'll find that higher concentrations react faster. The magnesium and hydrochloric acid variation lets you measure hydrogen gas production over time using an upturned measuring cylinder in water.
One limitation is that different people might see the cross disappear at slightly different times due to varying eyesight, but using the same size cross for everyone helps minimise this issue.
Practical Tip: Keep the same person doing the observations for all repeats to maintain consistency in your results.

Chromatography is your secret weapon for separating and identifying different chemicals in mixtures - think CSI but for food colourings! This technique works because different substances move up paper at different rates.
Draw a pencil line 2 cm from the bottom of your chromatography paper (pencil won't interfere with results like pen would). Use a capillary tube to place tiny spots of your food colourings on this line, keeping them small to prevent overlap.
Set up your paper so it dips into water (the solvent) but keep the pencil line above water level. As water moves up the paper, it carries the different dyes with it at different speeds. Mark where the solvent reaches before removing the paper.
Calculate Rf values by dividing the distance each spot moved by the distance the solvent moved. These values act like fingerprints - you can look them up in databases to identify unknown chemicals.
Why It Works: Different chemicals have different attractions to the paper and solvent, so they separate out as they move up together.
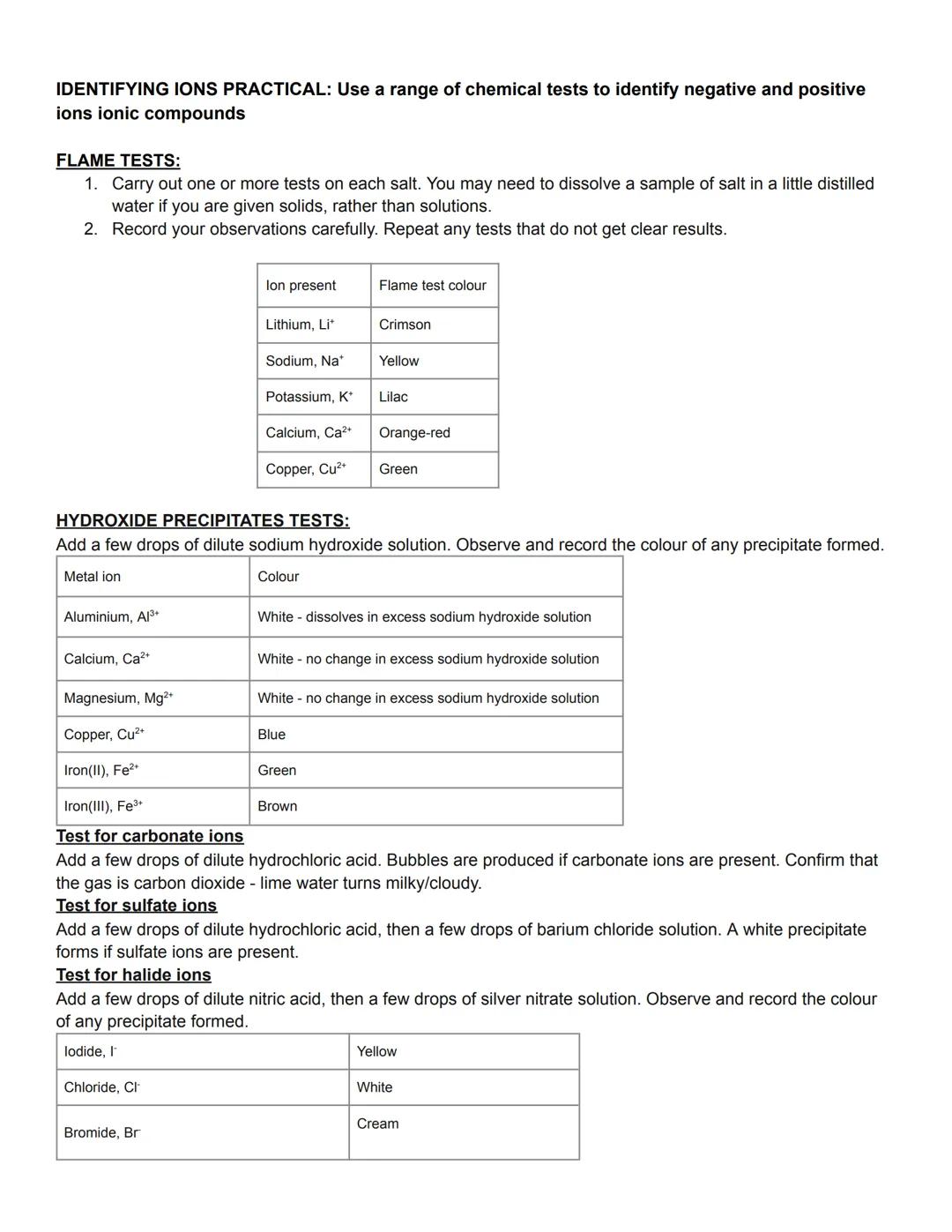
Ion identification is like being a chemical detective - you run specific tests and use the results to work out which ions are present. These tests are dead reliable and you'll definitely need them for exam questions.
Flame tests are brilliant for identifying metal ions - just dip a clean wire loop in your sample and hold it in a Bunsen flame. Lithium gives crimson, sodium gives yellow, and potassium gives lilac colours. Calcium produces orange-red whilst copper gives green.
For hydroxide precipitate tests, add sodium hydroxide solution and observe any solid that forms. Copper ions give blue precipitates, iron(II) gives green, and iron(III) gives brown. Aluminium, calcium, and magnesium all give white precipitates, but aluminium dissolves in excess sodium hydroxide.
Anion tests are equally straightforward - carbonates fizz with dilute hydrochloric acid (test the gas with lime water), sulfates give white precipitates with barium chloride, and halides give coloured precipitates with silver nitrate.
Memory Trick: For halides, remember "White Cream Yellow" - chloride (white), bromide (cream), iodide (yellow).

Water purity testing is more relevant than ever, and this practical teaches you exactly how to check if water is truly pure. Pure water should have a pH of 7 and contain no dissolved solids.
Test pH using universal indicator paper - it turns green for pH 7. For dissolved solids, weigh an empty evaporating basin, add your water sample, then heat until all water evaporates. If the basin's mass increases after cooling, you've got dissolved solids forming crystals on the surface.
Distillation purifies water by separating it from everything else. Heat your water sample in a conical flask connected to a delivery tube that leads into a cold test tube surrounded by ice. The water evaporates, travels along the tube as vapour, then condenses back into pure liquid water.
This distilled water should have pH 7 and leave no residue when evaporated - that's how you know it's genuinely pure. It's the same principle used in industrial water purification.
Real World: This distillation setup is basically a mini version of what happens in water treatment plants and even whisky distilleries!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Nema x friday
@nemaxfriday
Chemistry practicals are essential for your GCSE course, giving you hands-on experience with real chemical reactions. These eight required practicals cover everything from making salts to testing water purity, and you'll definitely see questions about them in your exams.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered how to make those brilliant blue crystals you see in chemistry labs? This practical shows you exactly how to prepare copper sulfate from copper oxide - and it's surprisingly straightforward once you know the steps.
You start with a fixed volume of sulfuric acid as your limiting reactant, then heat it until boiling. The clever bit is adding copper oxide bit by bit whilst stirring - you'll know it's working when the solution turns that gorgeous blue colour. Keep adding copper oxide until some powder remains unreacted, which tells you all the acid has been used up.
The final steps involve filtration to remove excess copper oxide, then gentle heating in an evaporating basin until half the solution remains. Leave it for 24 hours and you'll have proper copper sulfate crystals to scrape up and dry.
Top Tip: Always add the copper oxide gradually - if you dump it all in at once, the reaction might be too vigorous and you could miss the endpoint!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Titration is your go-to method for finding unknown concentrations, and this practical uses it to work out exactly how much acid neutralises a known amount of alkali. You'll be using this technique loads in A-levels, so getting comfortable with it now is brilliant.
Start by using a pipette to measure exactly 25 cm³ of sodium hydroxide into a conical flask - the conical shape reduces splashing risk. Add a few drops of phenolphthalein indicator, which stays pink in alkaline solutions but turns colourless when neutral.
Fill your burette with the unknown acid and slowly add it whilst swirling the flask. Once you see the colour starting to change, go drop by drop until you hit the end point - that magic moment when it goes from pink to completely clear. Always read the burette at eye level, using the bottom of the meniscus for accuracy.
Remember: The first titration is just a rough run - you'll need to repeat it several times to get consistent results for your calculations.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrolysis might seem complex, but this practical breaks it down into manageable chunks using two different solutions. You're essentially using electricity to split compounds apart, and different ions behave in predictable ways.
For copper chloride solution, set up your carbon electrodes in a petri dish with holes, making sure they don't touch (short circuits are bad news!). At 4V, you'll see brown copper forming at the cathode (negative electrode) because copper is less reactive than hydrogen. The anode produces chlorine gas - you can test this with blue litmus paper, which gets bleached.
Sodium chloride gives different results because sodium is more reactive than hydrogen. This means hydrogen gas bubbles off at the cathode instead of sodium metal, whilst chlorine still forms at the anode. You can test the hydrogen with a lit splint - it makes that classic squeaky pop sound.
Safety Note: Always work in a well-ventilated area when producing chlorine gas, and never let the electrodes touch each other!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This practical demonstrates exothermic reactions perfectly, showing how neutralisation between hydrochloric acid and sodium hydroxide releases energy as heat. You'll see exactly how changing reactant amounts affects temperature rise.
Use a polystyrene cup inside a beaker for insulation, starting with 30 cm³ of hydrochloric acid. Measure its temperature, then add 5 cm³ of sodium hydroxide solution through a plastic lid with your thermometer poking through. Stir gently and record the maximum temperature reached.
Repeat with increasing volumes of sodium hydroxide (10 cm³, 15 cm³, etc.) and you'll notice the temperature rises as more reactants are available. However, there's a twist - beyond a certain point, the temperature actually starts dropping again.
This happens because you've got excess sodium hydroxide that can't react, plus the energy released gets spread through a larger volume of solution. It's a brilliant example of how limiting reactants work in practice.
Key Point: The polystyrene cup and lid aren't just random equipment choices - they're essential for reducing heat loss and getting accurate results.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Rate of reaction practicals are fantastic for understanding how concentration affects how fast chemical changes happen. This one uses the classic disappearing cross method with sodium thiosulfate and hydrochloric acid.
The reaction produces sulfur as a cloudy precipitate, so you time how long it takes for a black cross underneath your flask to disappear. Start with 10 cm³ of each reactant, mix them quickly, and start your stopwatch immediately. When you can no longer see the cross, record the time and temperature.
Repeat with different concentrations of sodium thiosulfate - you'll find that higher concentrations react faster. The magnesium and hydrochloric acid variation lets you measure hydrogen gas production over time using an upturned measuring cylinder in water.
One limitation is that different people might see the cross disappear at slightly different times due to varying eyesight, but using the same size cross for everyone helps minimise this issue.
Practical Tip: Keep the same person doing the observations for all repeats to maintain consistency in your results.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chromatography is your secret weapon for separating and identifying different chemicals in mixtures - think CSI but for food colourings! This technique works because different substances move up paper at different rates.
Draw a pencil line 2 cm from the bottom of your chromatography paper (pencil won't interfere with results like pen would). Use a capillary tube to place tiny spots of your food colourings on this line, keeping them small to prevent overlap.
Set up your paper so it dips into water (the solvent) but keep the pencil line above water level. As water moves up the paper, it carries the different dyes with it at different speeds. Mark where the solvent reaches before removing the paper.
Calculate Rf values by dividing the distance each spot moved by the distance the solvent moved. These values act like fingerprints - you can look them up in databases to identify unknown chemicals.
Why It Works: Different chemicals have different attractions to the paper and solvent, so they separate out as they move up together.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ion identification is like being a chemical detective - you run specific tests and use the results to work out which ions are present. These tests are dead reliable and you'll definitely need them for exam questions.
Flame tests are brilliant for identifying metal ions - just dip a clean wire loop in your sample and hold it in a Bunsen flame. Lithium gives crimson, sodium gives yellow, and potassium gives lilac colours. Calcium produces orange-red whilst copper gives green.
For hydroxide precipitate tests, add sodium hydroxide solution and observe any solid that forms. Copper ions give blue precipitates, iron(II) gives green, and iron(III) gives brown. Aluminium, calcium, and magnesium all give white precipitates, but aluminium dissolves in excess sodium hydroxide.
Anion tests are equally straightforward - carbonates fizz with dilute hydrochloric acid (test the gas with lime water), sulfates give white precipitates with barium chloride, and halides give coloured precipitates with silver nitrate.
Memory Trick: For halides, remember "White Cream Yellow" - chloride (white), bromide (cream), iodide (yellow).

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Water purity testing is more relevant than ever, and this practical teaches you exactly how to check if water is truly pure. Pure water should have a pH of 7 and contain no dissolved solids.
Test pH using universal indicator paper - it turns green for pH 7. For dissolved solids, weigh an empty evaporating basin, add your water sample, then heat until all water evaporates. If the basin's mass increases after cooling, you've got dissolved solids forming crystals on the surface.
Distillation purifies water by separating it from everything else. Heat your water sample in a conical flask connected to a delivery tube that leads into a cold test tube surrounded by ice. The water evaporates, travels along the tube as vapour, then condenses back into pure liquid water.
This distilled water should have pH 7 and leave no residue when evaporated - that's how you know it's genuinely pure. It's the same principle used in industrial water purification.
Real World: This distillation setup is basically a mini version of what happens in water treatment plants and even whisky distilleries!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
1
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user