Ever wondered what makes up everything around you? It all... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
144
•
6 Feb 2026
•
G
@gurneet
Ever wondered what makes up everything around you? It all... Show more









Everything you see is made of atoms - literally the smallest pieces of elements that can exist. Think of them like LEGO blocks that build everything in the universe! There are roughly 100 different elements, each with their own unique type of atom.
Compounds form when elements chemically react and stick together in fixed amounts. Unlike mixing ingredients for a cake, these elements are properly bonded and you need chemical reactions to separate them again. Mixtures, on the other hand, are just different substances hanging out together without bonding - like a fruit salad where you can still pick out individual pieces.
The brilliant thing about mixtures is that you can separate them using physical methods. Filtration catches solids whilst letting liquids through (think coffee filter). Crystallisation evaporates the liquid to leave solid crystals behind, like getting salt from seawater. Simple distillation heats a solution so the liquid evaporates and condenses elsewhere, giving you pure liquid back.
Remember: Physical separation methods don't create new substances - they just separate what's already there!

Fractional distillation is like simple distillation's clever older sibling. It separates multiple liquids with different boiling points using a special column that ensures substances with lower boiling points escape first. This is exactly how crude oil gets refined into petrol, diesel, and other useful products.
Chromatography is your go-to method for separating dissolved substances like food dyes or inks. The sample travels up special paper with a solvent, and different substances travel different distances based on how much they "like" the paper versus the solvent.
Choosing the right separation method is dead simple: use filtration for insoluble solids, crystallisation for dissolved solids, distillation for different boiling points, and chromatography for analysing mixtures.
Scientists originally thought atoms were tiny, solid spheres that couldn't be broken down. The discovery of electrons changed everything, leading to the plum pudding model - imagine a ball of positive charge with negative electrons dotted throughout like raisins in a pudding.
Key insight: The alpha particle experiment proved atoms are mostly empty space with a tiny, dense, positive nucleus at the centre!

The nuclear model emerged when scientists realised that atoms have a small, dense, positive nucleus at the centre with electrons orbiting around it. Niels Bohr refined this by suggesting electrons orbit at specific distances, like planets around the sun but at set orbital paths.
Further discoveries revealed that the nucleus contains protons (positive charge) and neutrons (no charge), whilst electrons (negative charge) orbit outside. In any atom, the number of protons equals the number of electrons, making atoms electrically neutral overall.
Atoms are incredibly tiny - about 0.1 nanometres across. The nucleus is even smaller, being less than 1/10,000th the size of the whole atom, yet it contains almost all the atom's mass. Think of it like a marble in a football stadium!
The atomic number tells you how many protons an element has, whilst the mass number is the total of protons plus neutrons. Isotopes are atoms of the same element with different numbers of neutrons - they're like different versions of the same character in a video game.
Pro tip: Electrons have virtually no mass compared to protons and neutrons, so we often ignore them when calculating atomic mass!

Electrons arrange themselves in shells around the nucleus, filling the innermost shells first. Sodium's electronic structure is 2,8,1 - meaning 2 electrons in the first shell, 8 in the second, and 1 in the third. This arrangement determines how elements behave chemically.
The periodic table arranges elements by increasing atomic number (number of protons). Elements in the same vertical group have the same number of outer shell electrons, giving them similar chemical properties. The horizontal periods show how many electron shells an atom has.
Relative atomic mass accounts for all isotopes of an element. For chlorine with 75% chlorine-35 and 25% chlorine-37: Ar = (35×75 + 37×25) ÷ 100 = 35.5. This explains why atomic masses aren't always whole numbers on the periodic table.
The beauty of the periodic table is its predictive power - you can work out how many outer electrons an element has just from its group number, and how many shells it has from its period number.
Memory trick: Group number = outer electrons, Period number = number of shells!

The periodic table lets you predict how elements will behave. Group 1 metals get more reactive going down because their outer electrons are further from the nucleus and easier to lose. Group 7 halogens get less reactive going down because it's harder for larger atoms to attract extra electrons.
Group 0 noble gases are famously unreactive because they have full outer shells - they're chemically "satisfied" and don't need to react. Their boiling points increase down the group as the atoms get heavier.
Before we understood atomic structure, scientists like Mendeleev arranged elements by atomic weight and noticed patterns. He was clever enough to leave gaps for undiscovered elements and even predicted their properties. When these elements were found later, his predictions were spot-on!
Understanding isotopes later explained why strict atomic weight ordering sometimes failed. The modern periodic table uses atomic number instead, which works perfectly because it's based on the fundamental property of proton number.
Cool fact: Mendeleev's predictions were so accurate that when new elements were discovered, they fitted his gaps perfectly!

Metals are the shiny, strong materials that conduct electricity and can be hammered into shapes. They lose electrons in reactions to form positive ions. Most elements are metals, and they're generally found on the left side of the periodic table.
Non-metals are typically dull, brittle, and don't conduct electricity (except graphite). They gain or share electrons in reactions. The key difference is how they handle electrons during chemical reactions.
Group 1 alkali metals have one outer electron that's easily lost, making them highly reactive with water and forming alkaline solutions. Reactivity increases down the group as that outer electron gets further from the nucleus.
Group 7 halogens need one more electron to fill their outer shell, so they're eager to gain electrons. They become less reactive down the group because larger atoms find it harder to attract that extra electron.
Group 0 noble gases have complete outer shells (8 electrons, except helium with 2), making them incredibly stable and unreactive. They rarely form compounds because they're already "happy" chemically.
Think of it this way: Elements react to achieve full outer shells - metals lose electrons, non-metals gain them, and noble gases already have what they want!
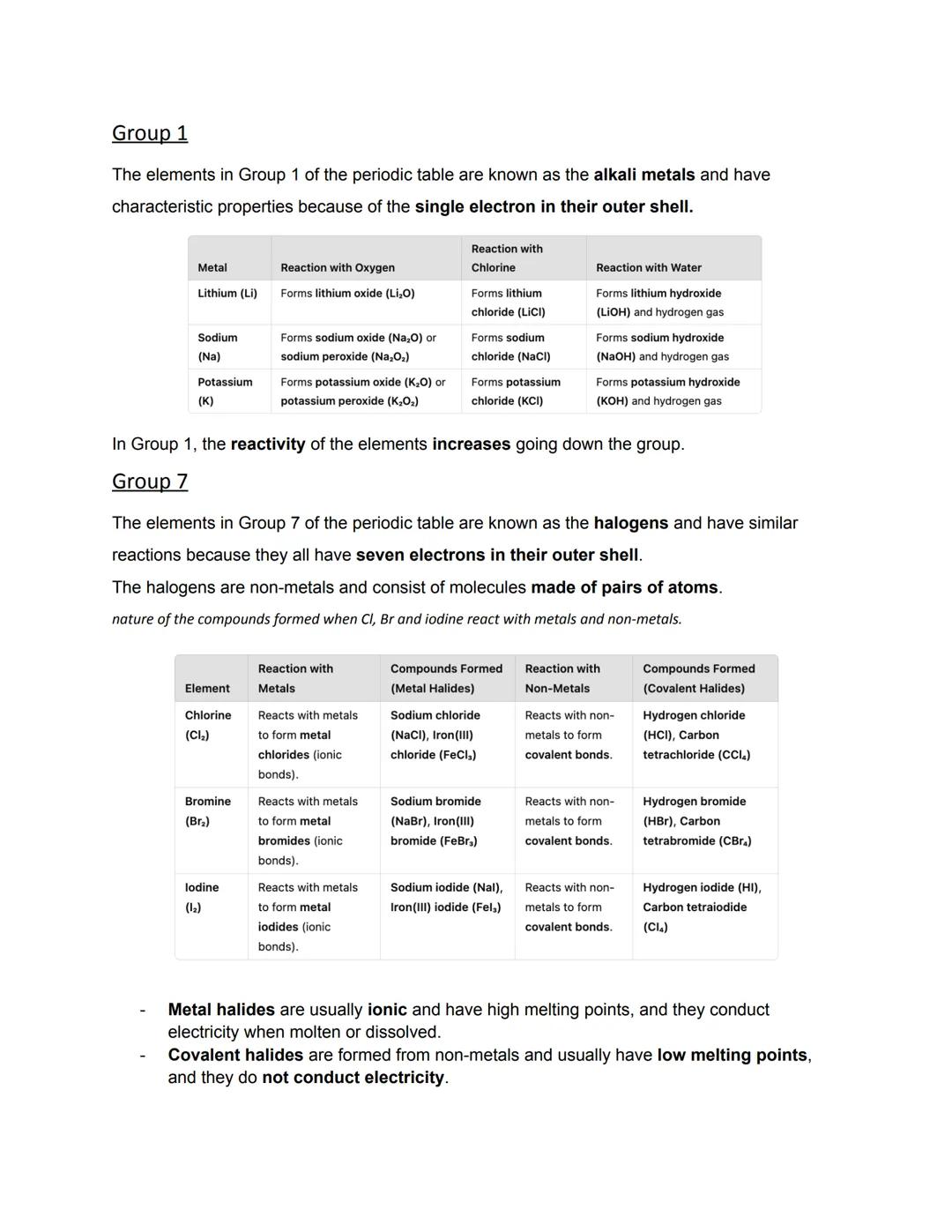
Group 1 metals all have one outer electron, making them incredibly reactive. They react with oxygen to form oxides, with chlorine to form chlorides, and with water to form hydroxides plus hydrogen gas. Lithium, sodium, and potassium all follow these patterns, but reactivity increases down the group.
When these metals hit water, they fizz about producing hydrogen gas and forming alkaline solutions. Potassium is so reactive it can even catch fire on water! This happens because the single outer electron becomes easier to lose as you go down the group.
Halogens exist as molecules of two atoms (like Cl₂, Br₂, I₂) and have seven outer electrons - just one short of a full shell. They react with metals to form ionic compounds (metal halides) and with non-metals to form covalent compounds.
Metal halides like sodium chloride have high melting points and conduct electricity when melted. Covalent halides like hydrogen chloride have low melting points and don't conduct electricity.
Moving down Group 7, the elements get heavier with higher melting and boiling points, but they become less reactive. More reactive halogens can push out less reactive ones from their compounds - chlorine can displace bromine, for example.
Displacement trick: A more reactive halogen will always kick out a less reactive one from its compound!

Transition metals are the tough guys of the periodic table - much harder, stronger, and denser than Group 1 metals. While sodium melts at just 98°C, iron doesn't melt until 1538°C! They're also much less reactive with water and oxygen.
These metals are incredibly useful because many form ions with different charges and create coloured compounds. Think of the blue-green colour of copper compounds or the rust-red of iron oxide. Many transition metals also work as catalysts, speeding up chemical reactions without being used up themselves.
The key difference between transition metals and Group 1 metals is stability. Transition metals are strong, dense, and relatively unreactive, making them perfect for construction and engineering. Group 1 metals are soft, light, and explosively reactive, so they're mainly used in compounds rather than as pure metals.
Whether you're looking at the steel in buildings or the copper in electrical wires, transition metals are everywhere because of their fantastic combination of strength and useful chemical properties.
Real-world connection: Your smartphone contains dozens of transition metals - from the copper in circuits to the rare earth elements in the screen!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
G
@gurneet
Ever wondered what makes up everything around you? It all starts with atoms - the tiny building blocks of matter. Understanding how atoms work and how elements are organised in the periodic table is the key to mastering chemistry.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Everything you see is made of atoms - literally the smallest pieces of elements that can exist. Think of them like LEGO blocks that build everything in the universe! There are roughly 100 different elements, each with their own unique type of atom.
Compounds form when elements chemically react and stick together in fixed amounts. Unlike mixing ingredients for a cake, these elements are properly bonded and you need chemical reactions to separate them again. Mixtures, on the other hand, are just different substances hanging out together without bonding - like a fruit salad where you can still pick out individual pieces.
The brilliant thing about mixtures is that you can separate them using physical methods. Filtration catches solids whilst letting liquids through (think coffee filter). Crystallisation evaporates the liquid to leave solid crystals behind, like getting salt from seawater. Simple distillation heats a solution so the liquid evaporates and condenses elsewhere, giving you pure liquid back.
Remember: Physical separation methods don't create new substances - they just separate what's already there!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Fractional distillation is like simple distillation's clever older sibling. It separates multiple liquids with different boiling points using a special column that ensures substances with lower boiling points escape first. This is exactly how crude oil gets refined into petrol, diesel, and other useful products.
Chromatography is your go-to method for separating dissolved substances like food dyes or inks. The sample travels up special paper with a solvent, and different substances travel different distances based on how much they "like" the paper versus the solvent.
Choosing the right separation method is dead simple: use filtration for insoluble solids, crystallisation for dissolved solids, distillation for different boiling points, and chromatography for analysing mixtures.
Scientists originally thought atoms were tiny, solid spheres that couldn't be broken down. The discovery of electrons changed everything, leading to the plum pudding model - imagine a ball of positive charge with negative electrons dotted throughout like raisins in a pudding.
Key insight: The alpha particle experiment proved atoms are mostly empty space with a tiny, dense, positive nucleus at the centre!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The nuclear model emerged when scientists realised that atoms have a small, dense, positive nucleus at the centre with electrons orbiting around it. Niels Bohr refined this by suggesting electrons orbit at specific distances, like planets around the sun but at set orbital paths.
Further discoveries revealed that the nucleus contains protons (positive charge) and neutrons (no charge), whilst electrons (negative charge) orbit outside. In any atom, the number of protons equals the number of electrons, making atoms electrically neutral overall.
Atoms are incredibly tiny - about 0.1 nanometres across. The nucleus is even smaller, being less than 1/10,000th the size of the whole atom, yet it contains almost all the atom's mass. Think of it like a marble in a football stadium!
The atomic number tells you how many protons an element has, whilst the mass number is the total of protons plus neutrons. Isotopes are atoms of the same element with different numbers of neutrons - they're like different versions of the same character in a video game.
Pro tip: Electrons have virtually no mass compared to protons and neutrons, so we often ignore them when calculating atomic mass!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrons arrange themselves in shells around the nucleus, filling the innermost shells first. Sodium's electronic structure is 2,8,1 - meaning 2 electrons in the first shell, 8 in the second, and 1 in the third. This arrangement determines how elements behave chemically.
The periodic table arranges elements by increasing atomic number (number of protons). Elements in the same vertical group have the same number of outer shell electrons, giving them similar chemical properties. The horizontal periods show how many electron shells an atom has.
Relative atomic mass accounts for all isotopes of an element. For chlorine with 75% chlorine-35 and 25% chlorine-37: Ar = (35×75 + 37×25) ÷ 100 = 35.5. This explains why atomic masses aren't always whole numbers on the periodic table.
The beauty of the periodic table is its predictive power - you can work out how many outer electrons an element has just from its group number, and how many shells it has from its period number.
Memory trick: Group number = outer electrons, Period number = number of shells!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The periodic table lets you predict how elements will behave. Group 1 metals get more reactive going down because their outer electrons are further from the nucleus and easier to lose. Group 7 halogens get less reactive going down because it's harder for larger atoms to attract extra electrons.
Group 0 noble gases are famously unreactive because they have full outer shells - they're chemically "satisfied" and don't need to react. Their boiling points increase down the group as the atoms get heavier.
Before we understood atomic structure, scientists like Mendeleev arranged elements by atomic weight and noticed patterns. He was clever enough to leave gaps for undiscovered elements and even predicted their properties. When these elements were found later, his predictions were spot-on!
Understanding isotopes later explained why strict atomic weight ordering sometimes failed. The modern periodic table uses atomic number instead, which works perfectly because it's based on the fundamental property of proton number.
Cool fact: Mendeleev's predictions were so accurate that when new elements were discovered, they fitted his gaps perfectly!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Metals are the shiny, strong materials that conduct electricity and can be hammered into shapes. They lose electrons in reactions to form positive ions. Most elements are metals, and they're generally found on the left side of the periodic table.
Non-metals are typically dull, brittle, and don't conduct electricity (except graphite). They gain or share electrons in reactions. The key difference is how they handle electrons during chemical reactions.
Group 1 alkali metals have one outer electron that's easily lost, making them highly reactive with water and forming alkaline solutions. Reactivity increases down the group as that outer electron gets further from the nucleus.
Group 7 halogens need one more electron to fill their outer shell, so they're eager to gain electrons. They become less reactive down the group because larger atoms find it harder to attract that extra electron.
Group 0 noble gases have complete outer shells (8 electrons, except helium with 2), making them incredibly stable and unreactive. They rarely form compounds because they're already "happy" chemically.
Think of it this way: Elements react to achieve full outer shells - metals lose electrons, non-metals gain them, and noble gases already have what they want!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Group 1 metals all have one outer electron, making them incredibly reactive. They react with oxygen to form oxides, with chlorine to form chlorides, and with water to form hydroxides plus hydrogen gas. Lithium, sodium, and potassium all follow these patterns, but reactivity increases down the group.
When these metals hit water, they fizz about producing hydrogen gas and forming alkaline solutions. Potassium is so reactive it can even catch fire on water! This happens because the single outer electron becomes easier to lose as you go down the group.
Halogens exist as molecules of two atoms (like Cl₂, Br₂, I₂) and have seven outer electrons - just one short of a full shell. They react with metals to form ionic compounds (metal halides) and with non-metals to form covalent compounds.
Metal halides like sodium chloride have high melting points and conduct electricity when melted. Covalent halides like hydrogen chloride have low melting points and don't conduct electricity.
Moving down Group 7, the elements get heavier with higher melting and boiling points, but they become less reactive. More reactive halogens can push out less reactive ones from their compounds - chlorine can displace bromine, for example.
Displacement trick: A more reactive halogen will always kick out a less reactive one from its compound!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Transition metals are the tough guys of the periodic table - much harder, stronger, and denser than Group 1 metals. While sodium melts at just 98°C, iron doesn't melt until 1538°C! They're also much less reactive with water and oxygen.
These metals are incredibly useful because many form ions with different charges and create coloured compounds. Think of the blue-green colour of copper compounds or the rust-red of iron oxide. Many transition metals also work as catalysts, speeding up chemical reactions without being used up themselves.
The key difference between transition metals and Group 1 metals is stability. Transition metals are strong, dense, and relatively unreactive, making them perfect for construction and engineering. Group 1 metals are soft, light, and explosively reactive, so they're mainly used in compounds rather than as pure metals.
Whether you're looking at the steel in buildings or the copper in electrical wires, transition metals are everywhere because of their fantastic combination of strength and useful chemical properties.
Real-world connection: Your smartphone contains dozens of transition metals - from the copper in circuits to the rare earth elements in the screen!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
2
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user