Chemical changes are happening all around you - from the... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
540
•
5 Feb 2026
•
Dae 🏎🪩
@d_h708
Chemical changes are happening all around you - from the... Show more






Ever wondered why some metals rust quickly whilst others stay shiny for years? It's all about reactivity - how eager metal atoms are to form positive ions when they react.
Metal oxides form when metals react with oxygen in oxidation reactions. Reactive metals like magnesium burn brilliantly in oxygen, whilst less reactive ones like iron just slowly tarnish without flames.
The reactivity series ranks metals from most to least reactive: potassium, sodium, calcium, magnesium, aluminium, zinc, iron, tin, copper. Remember it with: "Please Stop Calling My Aunty Zebra In The Class." Metals above hydrogen react with acids producing hydrogen gas - the more reactive, the more vigorous the fizzing.
Displacement reactions happen when a more reactive metal kicks out a less reactive one from its compound. Pop some magnesium into copper sulphate solution and watch the copper form as the magnesium takes its place. Try copper in zinc sulphate though, and nothing happens - copper isn't reactive enough to displace zinc.
Key Point: Only metals above hydrogen in the reactivity series will react with acids to produce hydrogen gas.

Here's where chemistry gets exciting - oxidation means losing electrons, whilst reduction means gaining them. Remember "OIL RIG": Oxidation Is Loss, Reduction Is Gain.
When zinc displaces copper from copper sulphate, zinc loses electrons (oxidised) whilst copper ions gain electrons (reduced). You can write these as half equations: Zn → Zn²⁺ + 2e⁻ and Cu²⁺ + 2e⁻ → Cu.
Acids react with metals above hydrogen to produce salts and hydrogen gas. Whether you get chlorides, nitrates, or sulphates depends on which acid you use - hydrochloric acid makes chlorides, nitric acid makes nitrates, sulphuric acid makes sulphates.
Neutralisation happens when acids meet bases or alkalis, producing salts and water. The key reaction is always H⁺ + OH⁻ → H₂O. Metal carbonates add extra excitement by producing carbon dioxide too - that's why limestone fizzes in acid rain.
Remember: All acid-metal reactions are redox reactions where the metal gets oxidised and hydrogen gets reduced.

The pH scale from 0-14 tells you how acidic or alkaline something is. Pure water sits neutrally at pH 7, acids are below 7, and alkalis are above 7. Universal indicator changes colour to show you exactly where your solution sits.
Strong acids like hydrochloric, nitric, and sulphuric acids completely break apart in water, releasing all their hydrogen ions. Weak acids like ethanoic acid (vinegar) only partially ionise - less than 1% of molecules actually release their hydrogen ions.
Don't confuse strong with concentrated! Concentrated just means lots of acid molecules per unit volume. You can have dilute strong acids and concentrated weak acids. When you dilute an acid 10 times, its pH increases by one unit.
Making soluble salts is straightforward - add solid base to acid until no more dissolves, filter off excess, then crystallise your salt solution. Just avoid metals that are too reactive (sodium) or too unreactive (copper).
Top Tip: As pH decreases by one unit, hydrogen ion concentration increases by a factor of 10 - that's why pH 2 is 10 times more acidic than pH 3.

Titrations let you find unknown concentrations by measuring exactly how much acid neutralises a known amount of alkali. You'll use a burette for the alkali, a pipette for precise acid measurement, and an indicator to spot the exact neutralisation point.
The method is all about precision - add alkali drop by drop near the end point, swirl constantly, and repeat until you get concordant results (within 0.1 cm³ of each other). Only average your concordant titres, never the dodgy first attempt.
Titration calculations follow three simple steps: calculate moles of the known substance, use the balanced equation to find moles of the unknown, then work out concentration. If 25 cm³ of HCl neutralises 22.4 cm³ of 2 mol/dm³ NaOH, you've got 0.0448 moles of NaOH, so 0.0448 moles of HCl too (1:1 ratio), giving 1.79 mol/dm³ HCl.
Remember your equipment - pipettes measure one fixed volume accurately, burettes measure variable volumes. Getting these mixed up in exams costs marks, so practise identifying them from diagrams.
Exam Success: Always convert cm³ to dm³ by dividing by 1000 before using the formula: moles = concentration × volume.

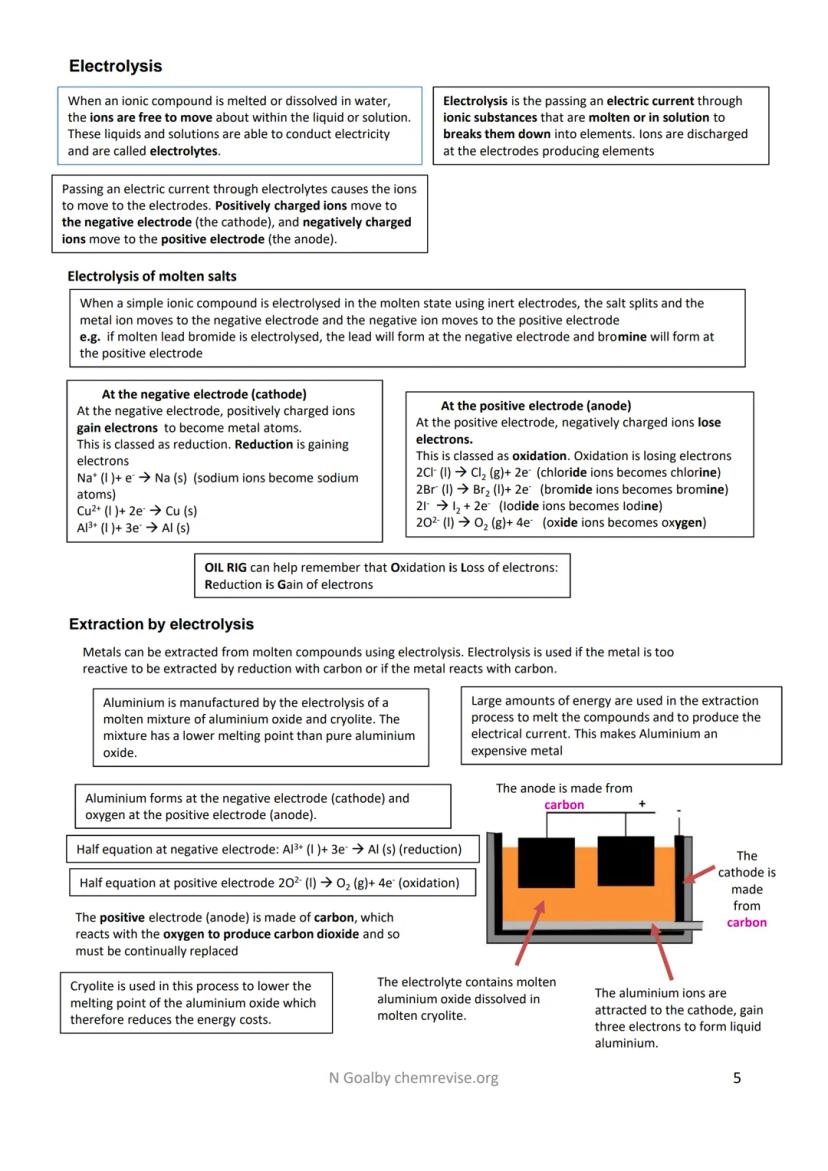
Electrolysis uses electricity to break apart ionic compounds when they're molten or dissolved. Think of it as forcing chemical reactions that wouldn't normally happen - you're literally pulling compounds apart with electrical power.
When you pass current through molten salts, positive ions head to the negative electrode (cathode) where they gain electrons and become metal atoms. Meanwhile, negative ions move to the positive electrode (anode) where they lose electrons to form non-metals.
Aluminium extraction showcases electrolysis perfectly. Aluminium oxide mixed with cryolite gets electrolysed - aluminium forms at the cathode, oxygen at the anode. The cryolite lowers the melting point, saving energy costs, but you still need massive amounts of electricity making aluminium expensive.
The carbon anodes keep getting eaten away because they react with the oxygen produced, forming carbon dioxide. That's why aluminium smelters constantly replace their anodes - it's an ongoing cost of the process.
Remember OIL RIG: At the anode, oxidation happens (ions lose electrons). At the cathode, reduction happens (ions gain electrons).
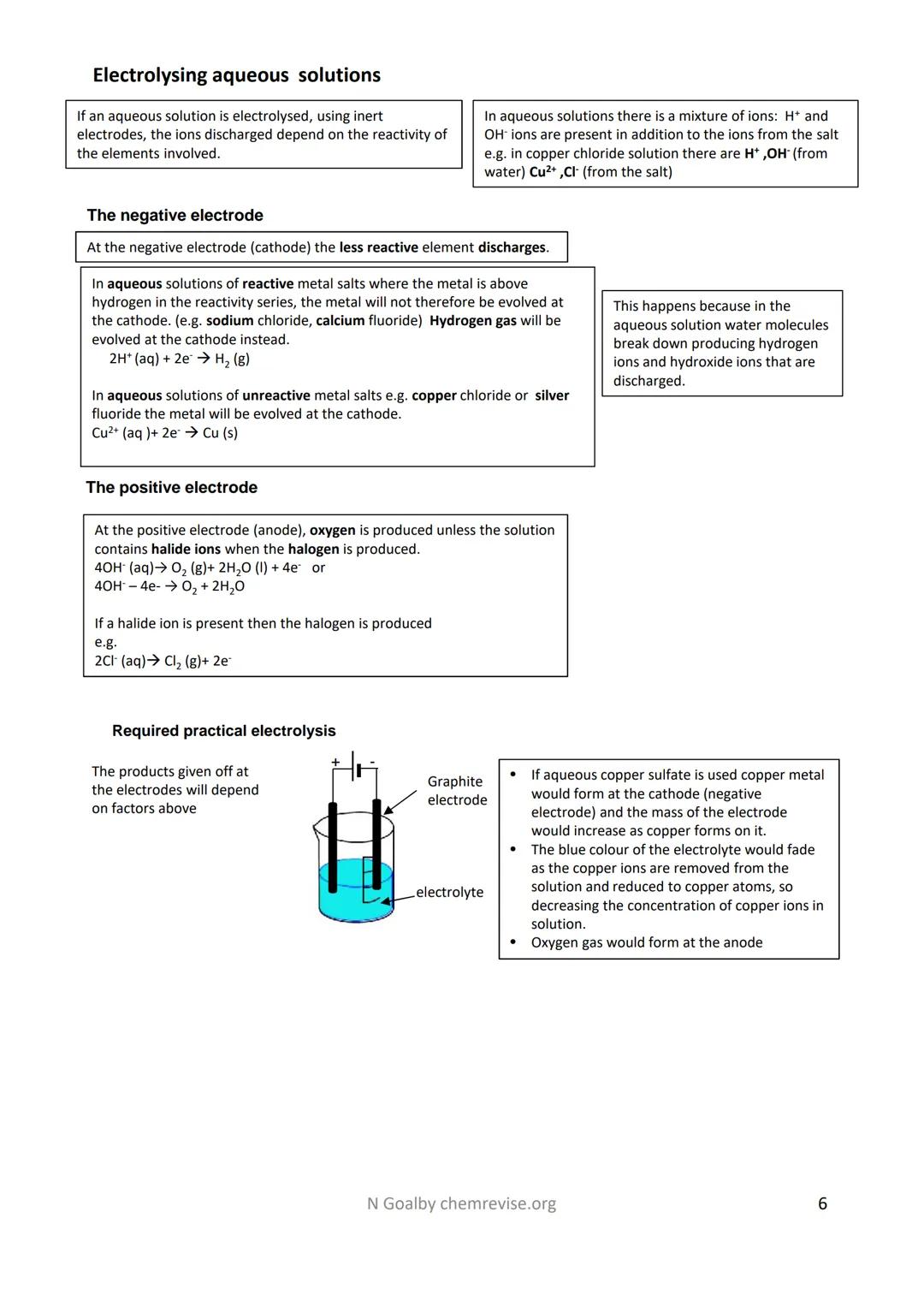
Aqueous solutions make electrolysis trickier because water adds H⁺ and OH⁻ ions to the mix. Now you've got competition - which ions actually get discharged at each electrode?
At the negative electrode (cathode), the less reactive element wins. If you electrolyse sodium chloride solution, hydrogen forms instead of sodium because sodium is too reactive. But with copper chloride solution, copper metal forms because copper is less reactive than hydrogen.
At the positive electrode (anode), oxygen usually forms from OH⁻ ions - unless halide ions are present. Then you get the halogen instead. Electrolyse sodium chloride and you'll get chlorine gas, not oxygen.
Watch for the visual clues in the required practical. If you electrolyse copper sulphate solution, the cathode gains mass as copper deposits on it, whilst the blue colour fades as copper ions get removed from solution. Meanwhile, oxygen bubbles form at the anode.
Quick Check: Reactive metals above hydrogen in the reactivity series won't form at the cathode in aqueous solutions - hydrogen will form instead.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Dae 🏎🪩
@d_h708
Chemical changes are happening all around you - from the rust on a bike to the fizzing when you drop antacid in water. Understanding how metals react, acids work, and electricity can break apart compounds gives you the tools to... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why some metals rust quickly whilst others stay shiny for years? It's all about reactivity - how eager metal atoms are to form positive ions when they react.
Metal oxides form when metals react with oxygen in oxidation reactions. Reactive metals like magnesium burn brilliantly in oxygen, whilst less reactive ones like iron just slowly tarnish without flames.
The reactivity series ranks metals from most to least reactive: potassium, sodium, calcium, magnesium, aluminium, zinc, iron, tin, copper. Remember it with: "Please Stop Calling My Aunty Zebra In The Class." Metals above hydrogen react with acids producing hydrogen gas - the more reactive, the more vigorous the fizzing.
Displacement reactions happen when a more reactive metal kicks out a less reactive one from its compound. Pop some magnesium into copper sulphate solution and watch the copper form as the magnesium takes its place. Try copper in zinc sulphate though, and nothing happens - copper isn't reactive enough to displace zinc.
Key Point: Only metals above hydrogen in the reactivity series will react with acids to produce hydrogen gas.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's where chemistry gets exciting - oxidation means losing electrons, whilst reduction means gaining them. Remember "OIL RIG": Oxidation Is Loss, Reduction Is Gain.
When zinc displaces copper from copper sulphate, zinc loses electrons (oxidised) whilst copper ions gain electrons (reduced). You can write these as half equations: Zn → Zn²⁺ + 2e⁻ and Cu²⁺ + 2e⁻ → Cu.
Acids react with metals above hydrogen to produce salts and hydrogen gas. Whether you get chlorides, nitrates, or sulphates depends on which acid you use - hydrochloric acid makes chlorides, nitric acid makes nitrates, sulphuric acid makes sulphates.
Neutralisation happens when acids meet bases or alkalis, producing salts and water. The key reaction is always H⁺ + OH⁻ → H₂O. Metal carbonates add extra excitement by producing carbon dioxide too - that's why limestone fizzes in acid rain.
Remember: All acid-metal reactions are redox reactions where the metal gets oxidised and hydrogen gets reduced.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The pH scale from 0-14 tells you how acidic or alkaline something is. Pure water sits neutrally at pH 7, acids are below 7, and alkalis are above 7. Universal indicator changes colour to show you exactly where your solution sits.
Strong acids like hydrochloric, nitric, and sulphuric acids completely break apart in water, releasing all their hydrogen ions. Weak acids like ethanoic acid (vinegar) only partially ionise - less than 1% of molecules actually release their hydrogen ions.
Don't confuse strong with concentrated! Concentrated just means lots of acid molecules per unit volume. You can have dilute strong acids and concentrated weak acids. When you dilute an acid 10 times, its pH increases by one unit.
Making soluble salts is straightforward - add solid base to acid until no more dissolves, filter off excess, then crystallise your salt solution. Just avoid metals that are too reactive (sodium) or too unreactive (copper).
Top Tip: As pH decreases by one unit, hydrogen ion concentration increases by a factor of 10 - that's why pH 2 is 10 times more acidic than pH 3.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Titrations let you find unknown concentrations by measuring exactly how much acid neutralises a known amount of alkali. You'll use a burette for the alkali, a pipette for precise acid measurement, and an indicator to spot the exact neutralisation point.
The method is all about precision - add alkali drop by drop near the end point, swirl constantly, and repeat until you get concordant results (within 0.1 cm³ of each other). Only average your concordant titres, never the dodgy first attempt.
Titration calculations follow three simple steps: calculate moles of the known substance, use the balanced equation to find moles of the unknown, then work out concentration. If 25 cm³ of HCl neutralises 22.4 cm³ of 2 mol/dm³ NaOH, you've got 0.0448 moles of NaOH, so 0.0448 moles of HCl too (1:1 ratio), giving 1.79 mol/dm³ HCl.
Remember your equipment - pipettes measure one fixed volume accurately, burettes measure variable volumes. Getting these mixed up in exams costs marks, so practise identifying them from diagrams.
Exam Success: Always convert cm³ to dm³ by dividing by 1000 before using the formula: moles = concentration × volume.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Electrolysis uses electricity to break apart ionic compounds when they're molten or dissolved. Think of it as forcing chemical reactions that wouldn't normally happen - you're literally pulling compounds apart with electrical power.
When you pass current through molten salts, positive ions head to the negative electrode (cathode) where they gain electrons and become metal atoms. Meanwhile, negative ions move to the positive electrode (anode) where they lose electrons to form non-metals.
Aluminium extraction showcases electrolysis perfectly. Aluminium oxide mixed with cryolite gets electrolysed - aluminium forms at the cathode, oxygen at the anode. The cryolite lowers the melting point, saving energy costs, but you still need massive amounts of electricity making aluminium expensive.
The carbon anodes keep getting eaten away because they react with the oxygen produced, forming carbon dioxide. That's why aluminium smelters constantly replace their anodes - it's an ongoing cost of the process.
Remember OIL RIG: At the anode, oxidation happens (ions lose electrons). At the cathode, reduction happens (ions gain electrons).

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Aqueous solutions make electrolysis trickier because water adds H⁺ and OH⁻ ions to the mix. Now you've got competition - which ions actually get discharged at each electrode?
At the negative electrode (cathode), the less reactive element wins. If you electrolyse sodium chloride solution, hydrogen forms instead of sodium because sodium is too reactive. But with copper chloride solution, copper metal forms because copper is less reactive than hydrogen.
At the positive electrode (anode), oxygen usually forms from OH⁻ ions - unless halide ions are present. Then you get the halogen instead. Electrolyse sodium chloride and you'll get chlorine gas, not oxygen.
Watch for the visual clues in the required practical. If you electrolyse copper sulphate solution, the cathode gains mass as copper deposits on it, whilst the blue colour fades as copper ions get removed from solution. Meanwhile, oxygen bubbles form at the anode.
Quick Check: Reactive metals above hydrogen in the reactivity series won't form at the cathode in aqueous solutions - hydrogen will form instead.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
11
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user