Ever wondered why some reactions make things hot whilst others... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
290
•
6 Feb 2026
•
Celine
@elineokiemutetobele_jzkp
Ever wondered why some reactions make things hot whilst others... Show more










Think about striking a match or using an instant ice pack - these are perfect examples of how reactions either release or absorb energy from their surroundings. The key thing to remember is that energy is always conserved during chemical reactions, meaning the total amount stays the same.
Exothermic reactions are the dramatic ones - they transfer energy to the surroundings, making things heat up. The product molecules end up with less energy than the reactants because they've given energy away. You'll see this happening during combustion (like burning fuel), oxidation (like rusting), and neutralisation reactions.
Endothermic reactions do the opposite - they're energy thieves that take heat from the surroundings, cooling things down. Here, the products have more energy than the reactants because they've absorbed it. Thermal decomposition is a classic example, like when calcium carbonate breaks down into calcium oxide and carbon dioxide when heated.
Quick Tip: Remember EXO = EXit (energy leaves), ENDO = ENter (energy enters)

Reaction profiles are like energy maps that show you the journey from reactants to products. They reveal something crucial: particles need a minimum amount of energy to react, called the activation energy - think of it as the energy hurdle they must jump over.
For exothermic reactions, the graph shows products sitting at a lower energy level than reactants. The difference in height tells you exactly how much energy gets released per mole. The initial energy spike shows the activation energy needed to get things started.
The higher the activation energy peak, the more energy you need to kick-start the reaction. This explains why some reactions happen easily at room temperature whilst others need heating up first.
Exam Tip: Always label the activation energy as the peak from reactants, not from zero!

Here's where chemistry gets mathematical - but in a useful way! Every chemical reaction involves breaking old bonds and forming new ones. Breaking bonds always requires energy (like snapping elastic bands), whilst forming bonds always releases energy.
The overall energy change depends on which process needs more energy. In exothermic reactions, more energy comes from making new bonds than breaking old ones. In endothermic reactions, it's the other way round.
You can calculate these changes using bond energies - specific values that tell you how much energy each type of bond needs. Different bonds have different strengths: H-H bonds need 432 kJ/mol to break, whilst C-Cl bonds only need 240 kJ/mol.
Calculation Reminder: Total energy change = Energy needed to break bonds - Energy released from making bonds
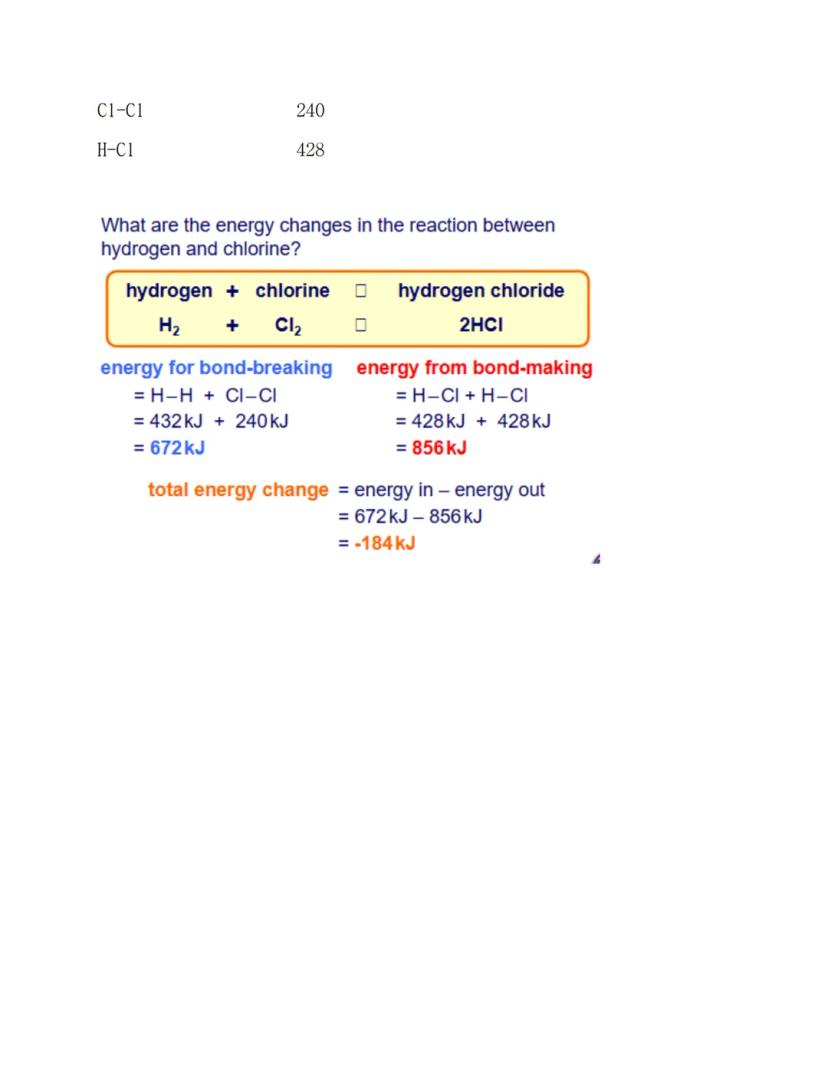
Let's see bond energy calculations in action with hydrogen and chlorine making hydrogen chloride. This systematic approach works for any reaction once you know the bond energies.
First, work out the energy needed for bond-breaking: H-H bond (432 kJ) plus Cl-Cl bond (240 kJ) gives 672 kJ total. Then calculate the energy from bond-making: two H-Cl bonds (428 kJ each) gives 856 kJ total.
The final step gives you the overall energy change: 672 kJ - 856 kJ = -184 kJ. The negative sign tells you this is exothermic - energy gets released to the surroundings.
Sign Check: Negative = exothermic (energy out), Positive = endothermic (energy in)

Calorimetry lets you measure exactly how much energy a reaction releases or absorbs. It's simpler than it sounds - you're basically taking the temperature before and after mixing reactants in an insulated cup.
The key challenge is preventing heat loss to the surroundings. That's why you use polystyrene cups (good insulators), surround them with cotton wool, and add lids. Every bit of insulation helps get more accurate results.
The method involves measuring equal volumes of reactants at the same starting temperature, mixing them quickly, and recording the highest temperature reached. You'll repeat this with different concentrations to see how it affects the energy change.
Safety Note: Always wear safety goggles when handling acids and alkalis, even dilute ones!

Hydrocarbons are chemistry's building blocks - simple compounds made from just carbon and hydrogen atoms. They're everywhere in your daily life, from the petrol in cars to the gas that heats your home.
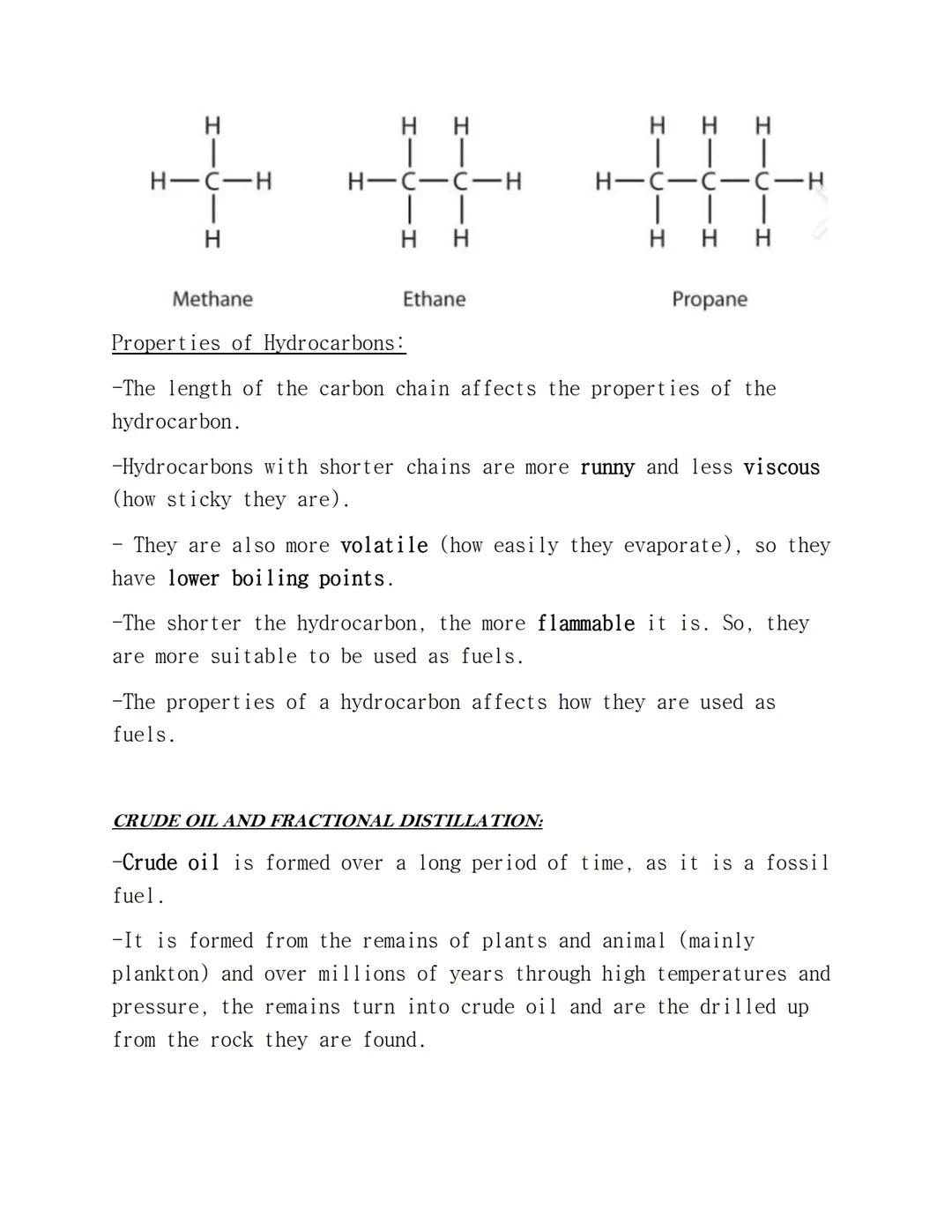
Alkanes are the simplest hydrocarbons with the general formula CnH2n+2. They're called saturated because each carbon forms four single bonds - no double bonds allowed. Being a homologous series means they all react in similar ways.
You need to know the first seven alkanes: methane (CH₄), ethane (C₂H₆), propane (C₃H₈), butane (C₄H₁₀), pentane (C₅H₁₂), hexane (C₆H₁₄), and heptane (C₇H₁₆). Notice how each one adds a CH₂ unit.
Memory Trick: "My Enormous Penguin Brings Presents Home Happily" for the first letters!
The length of hydrocarbon chains dramatically affects their properties. Shorter chains are runny (less viscous), evaporate easily (more volatile), have lower boiling points, and burn more readily. This makes them brilliant fuels.
Crude oil formed over millions of years from dead plants and animals (mainly plankton) that got buried under rock. High temperatures and pressure slowly transformed these remains into the mixture of hydrocarbons we drill up today.
Since crude oil contains hydrocarbons of many different lengths all mixed together, we need fractional distillation to separate them into useful fractions. Each fraction contains hydrocarbons with similar boiling points and chain lengths.
Time Scale: It takes millions of years to form crude oil - that's why it's called a fossil fuel!

Fractional distillation works by heating crude oil until it becomes gas, then letting different hydrocarbons condense at different temperatures. Longer chains (higher boiling points) condense first near the bottom, whilst shorter chains (lower boiling points) condense higher up where it's cooler.
The separated fractions become feedstock for making polymers, solvents, lubricants, and detergents. Carbon's ability to form long chains and branch in different ways creates this amazing variety of products.
Cracking solves a practical problem - fractional distillation produces too many long-chain alkanes and not enough short ones for fuel. This process breaks long chains into shorter, more useful pieces, also producing reactive alkenes as byproducts.
Economic Point: Supply and demand - we need more short chains than crude oil naturally provides!

Cracking is essentially thermal decomposition - using heat to break down molecules. Catalytic cracking vaporises long hydrocarbons and passes them over hot aluminium oxide catalyst. Steam cracking mixes the vapours with steam at very high temperatures.
The products include useful shorter alkanes for fuel, plus alkenes with the general formula CnH2n. Alkenes are much more reactive than alkanes, making them perfect starting materials for other compounds and polymers.
You can easily test for alkenes using bromine water - it stays orange with alkanes (no reaction) but turns colourless with alkenes. This colour change happens because bromine reacts with the double bond in alkenes.
Lab Test: Orange to colourless = alkene present. No colour change = alkane.

When hydrocarbons burn, what you get depends on how much oxygen is available. Complete combustion happens with plenty of oxygen around - you get just carbon dioxide and water as products. The equation is simple: hydrocarbon + oxygen → carbon dioxide + water.
Incomplete combustion occurs when oxygen supply is limited, producing different and often dangerous products. This is why proper ventilation matters when burning fuels - you want complete combustion for safety and efficiency.
Understanding combustion is crucial because it's how we get energy from fossil fuels. Complete combustion releases more energy and produces cleaner products than incomplete combustion.
Safety Alert: Incomplete combustion can produce toxic carbon monoxide - always ensure good ventilation!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Celine
@elineokiemutetobele_jzkp
Ever wondered why some reactions make things hot whilst others cool them down? Chemical reactions are all about energy changes, and understanding these patterns will help you predict what happens when different substances react together.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think about striking a match or using an instant ice pack - these are perfect examples of how reactions either release or absorb energy from their surroundings. The key thing to remember is that energy is always conserved during chemical reactions, meaning the total amount stays the same.
Exothermic reactions are the dramatic ones - they transfer energy to the surroundings, making things heat up. The product molecules end up with less energy than the reactants because they've given energy away. You'll see this happening during combustion (like burning fuel), oxidation (like rusting), and neutralisation reactions.
Endothermic reactions do the opposite - they're energy thieves that take heat from the surroundings, cooling things down. Here, the products have more energy than the reactants because they've absorbed it. Thermal decomposition is a classic example, like when calcium carbonate breaks down into calcium oxide and carbon dioxide when heated.
Quick Tip: Remember EXO = EXit (energy leaves), ENDO = ENter (energy enters)

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Reaction profiles are like energy maps that show you the journey from reactants to products. They reveal something crucial: particles need a minimum amount of energy to react, called the activation energy - think of it as the energy hurdle they must jump over.
For exothermic reactions, the graph shows products sitting at a lower energy level than reactants. The difference in height tells you exactly how much energy gets released per mole. The initial energy spike shows the activation energy needed to get things started.
The higher the activation energy peak, the more energy you need to kick-start the reaction. This explains why some reactions happen easily at room temperature whilst others need heating up first.
Exam Tip: Always label the activation energy as the peak from reactants, not from zero!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Here's where chemistry gets mathematical - but in a useful way! Every chemical reaction involves breaking old bonds and forming new ones. Breaking bonds always requires energy (like snapping elastic bands), whilst forming bonds always releases energy.
The overall energy change depends on which process needs more energy. In exothermic reactions, more energy comes from making new bonds than breaking old ones. In endothermic reactions, it's the other way round.
You can calculate these changes using bond energies - specific values that tell you how much energy each type of bond needs. Different bonds have different strengths: H-H bonds need 432 kJ/mol to break, whilst C-Cl bonds only need 240 kJ/mol.
Calculation Reminder: Total energy change = Energy needed to break bonds - Energy released from making bonds

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
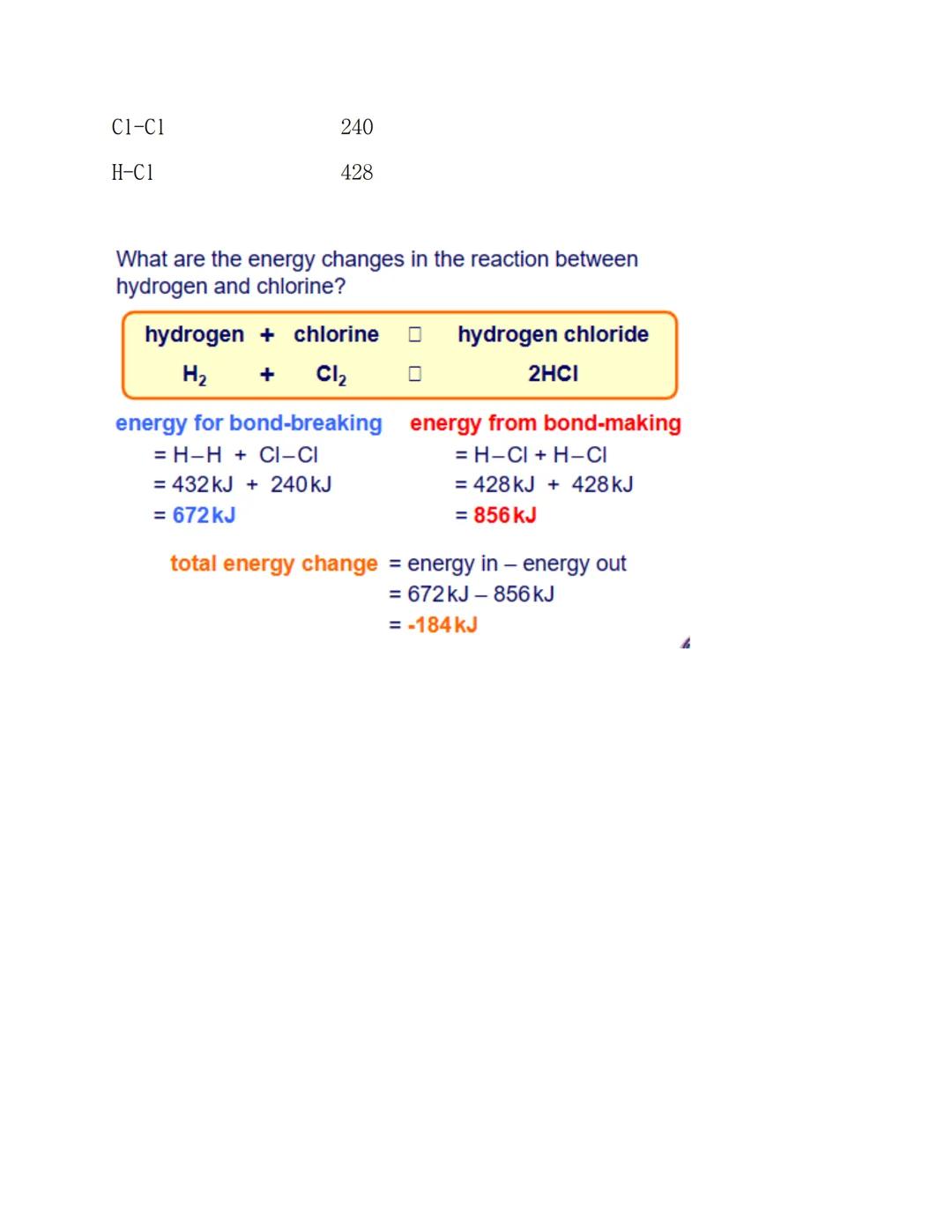
Let's see bond energy calculations in action with hydrogen and chlorine making hydrogen chloride. This systematic approach works for any reaction once you know the bond energies.
First, work out the energy needed for bond-breaking: H-H bond (432 kJ) plus Cl-Cl bond (240 kJ) gives 672 kJ total. Then calculate the energy from bond-making: two H-Cl bonds (428 kJ each) gives 856 kJ total.
The final step gives you the overall energy change: 672 kJ - 856 kJ = -184 kJ. The negative sign tells you this is exothermic - energy gets released to the surroundings.
Sign Check: Negative = exothermic (energy out), Positive = endothermic (energy in)

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Calorimetry lets you measure exactly how much energy a reaction releases or absorbs. It's simpler than it sounds - you're basically taking the temperature before and after mixing reactants in an insulated cup.
The key challenge is preventing heat loss to the surroundings. That's why you use polystyrene cups (good insulators), surround them with cotton wool, and add lids. Every bit of insulation helps get more accurate results.
The method involves measuring equal volumes of reactants at the same starting temperature, mixing them quickly, and recording the highest temperature reached. You'll repeat this with different concentrations to see how it affects the energy change.
Safety Note: Always wear safety goggles when handling acids and alkalis, even dilute ones!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Hydrocarbons are chemistry's building blocks - simple compounds made from just carbon and hydrogen atoms. They're everywhere in your daily life, from the petrol in cars to the gas that heats your home.
Alkanes are the simplest hydrocarbons with the general formula CnH2n+2. They're called saturated because each carbon forms four single bonds - no double bonds allowed. Being a homologous series means they all react in similar ways.
You need to know the first seven alkanes: methane (CH₄), ethane (C₂H₆), propane (C₃H₈), butane (C₄H₁₀), pentane (C₅H₁₂), hexane (C₆H₁₄), and heptane (C₇H₁₆). Notice how each one adds a CH₂ unit.
Memory Trick: "My Enormous Penguin Brings Presents Home Happily" for the first letters!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The length of hydrocarbon chains dramatically affects their properties. Shorter chains are runny (less viscous), evaporate easily (more volatile), have lower boiling points, and burn more readily. This makes them brilliant fuels.
Crude oil formed over millions of years from dead plants and animals (mainly plankton) that got buried under rock. High temperatures and pressure slowly transformed these remains into the mixture of hydrocarbons we drill up today.
Since crude oil contains hydrocarbons of many different lengths all mixed together, we need fractional distillation to separate them into useful fractions. Each fraction contains hydrocarbons with similar boiling points and chain lengths.
Time Scale: It takes millions of years to form crude oil - that's why it's called a fossil fuel!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Fractional distillation works by heating crude oil until it becomes gas, then letting different hydrocarbons condense at different temperatures. Longer chains (higher boiling points) condense first near the bottom, whilst shorter chains (lower boiling points) condense higher up where it's cooler.
The separated fractions become feedstock for making polymers, solvents, lubricants, and detergents. Carbon's ability to form long chains and branch in different ways creates this amazing variety of products.
Cracking solves a practical problem - fractional distillation produces too many long-chain alkanes and not enough short ones for fuel. This process breaks long chains into shorter, more useful pieces, also producing reactive alkenes as byproducts.
Economic Point: Supply and demand - we need more short chains than crude oil naturally provides!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Cracking is essentially thermal decomposition - using heat to break down molecules. Catalytic cracking vaporises long hydrocarbons and passes them over hot aluminium oxide catalyst. Steam cracking mixes the vapours with steam at very high temperatures.
The products include useful shorter alkanes for fuel, plus alkenes with the general formula CnH2n. Alkenes are much more reactive than alkanes, making them perfect starting materials for other compounds and polymers.
You can easily test for alkenes using bromine water - it stays orange with alkanes (no reaction) but turns colourless with alkenes. This colour change happens because bromine reacts with the double bond in alkenes.
Lab Test: Orange to colourless = alkene present. No colour change = alkane.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When hydrocarbons burn, what you get depends on how much oxygen is available. Complete combustion happens with plenty of oxygen around - you get just carbon dioxide and water as products. The equation is simple: hydrocarbon + oxygen → carbon dioxide + water.
Incomplete combustion occurs when oxygen supply is limited, producing different and often dangerous products. This is why proper ventilation matters when burning fuels - you want complete combustion for safety and efficiency.
Understanding combustion is crucial because it's how we get energy from fossil fuels. Complete combustion releases more energy and produces cleaner products than incomplete combustion.
Safety Alert: Incomplete combustion can produce toxic carbon monoxide - always ensure good ventilation!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
5
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user