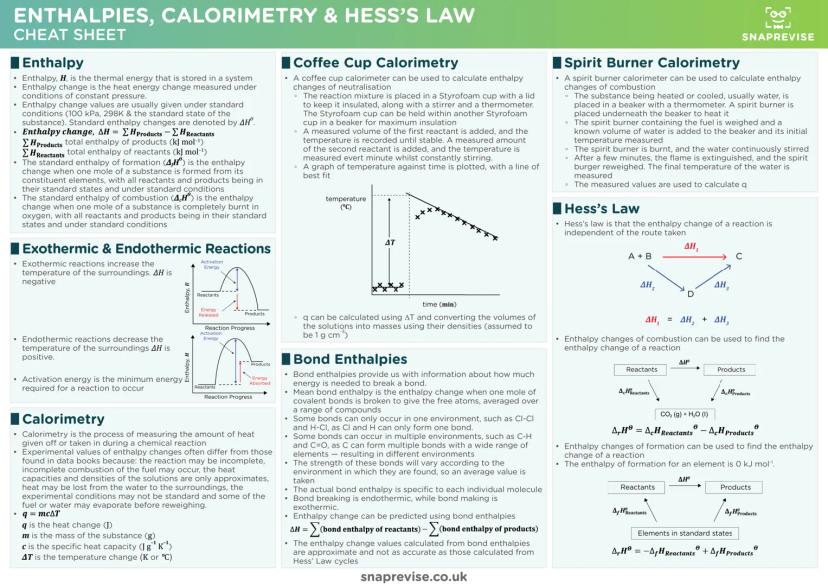
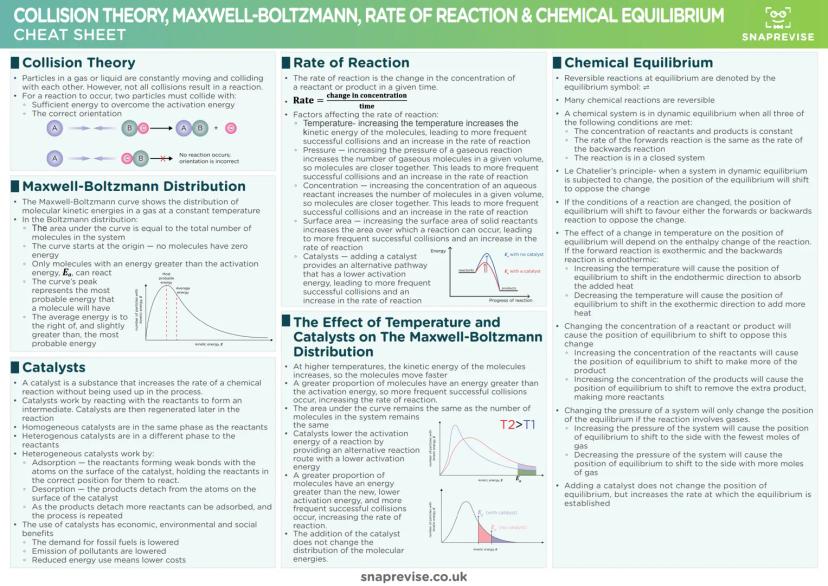
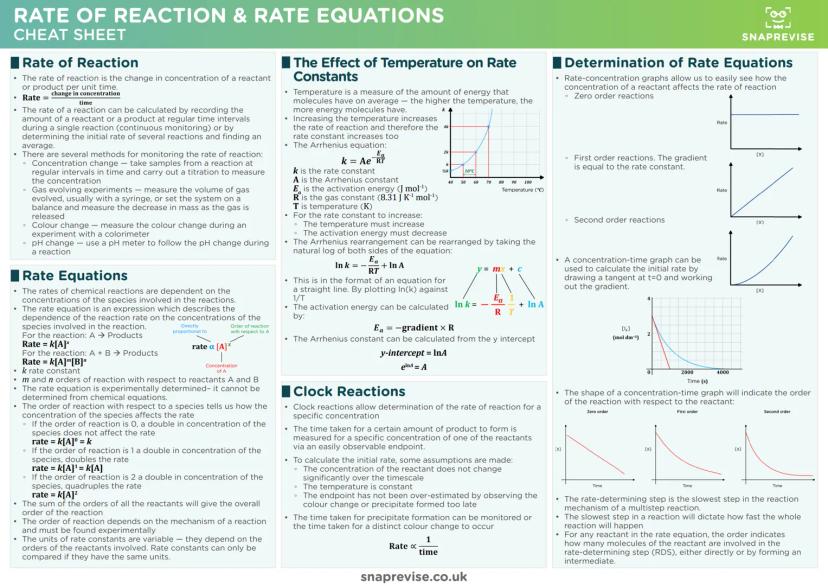
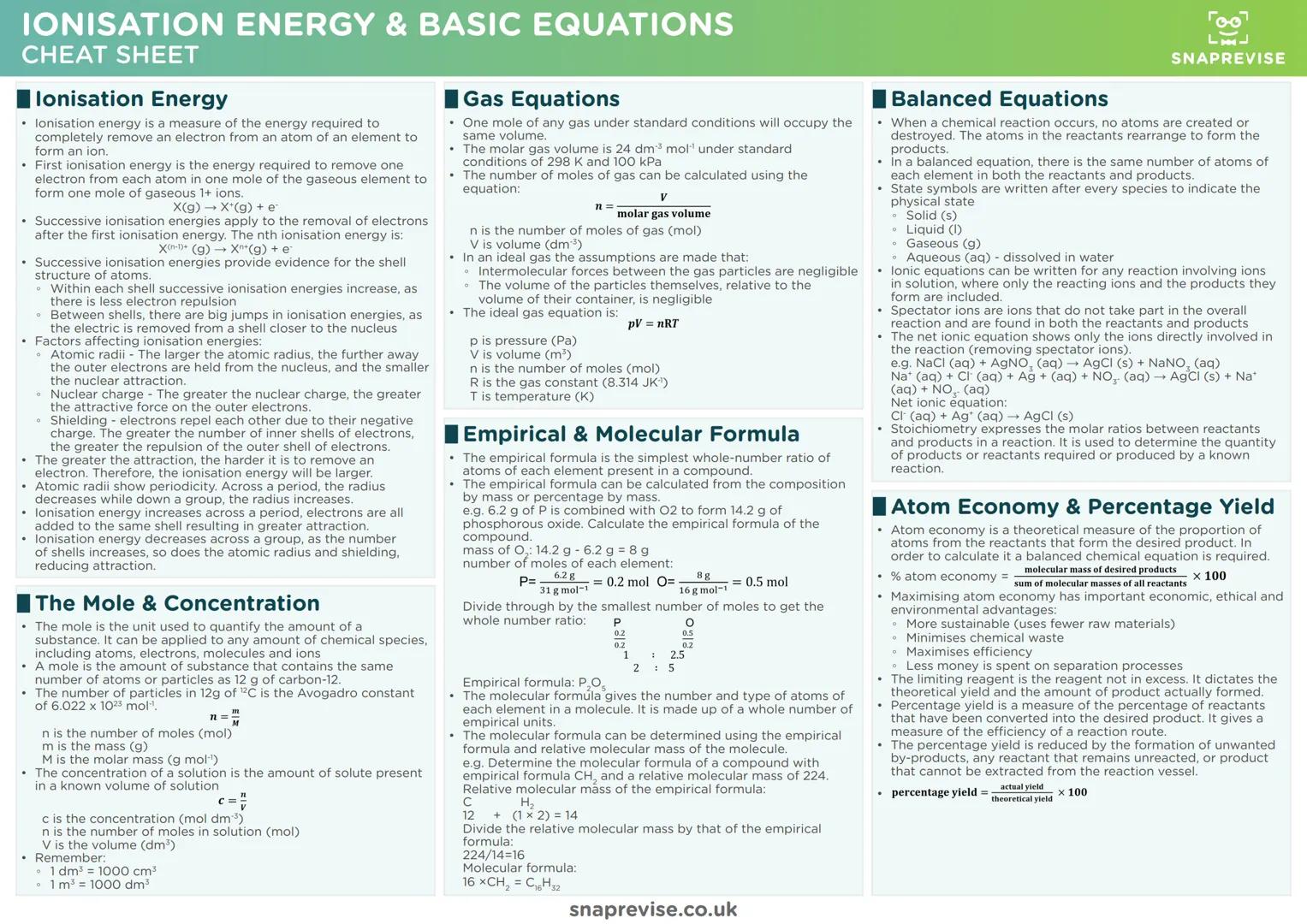
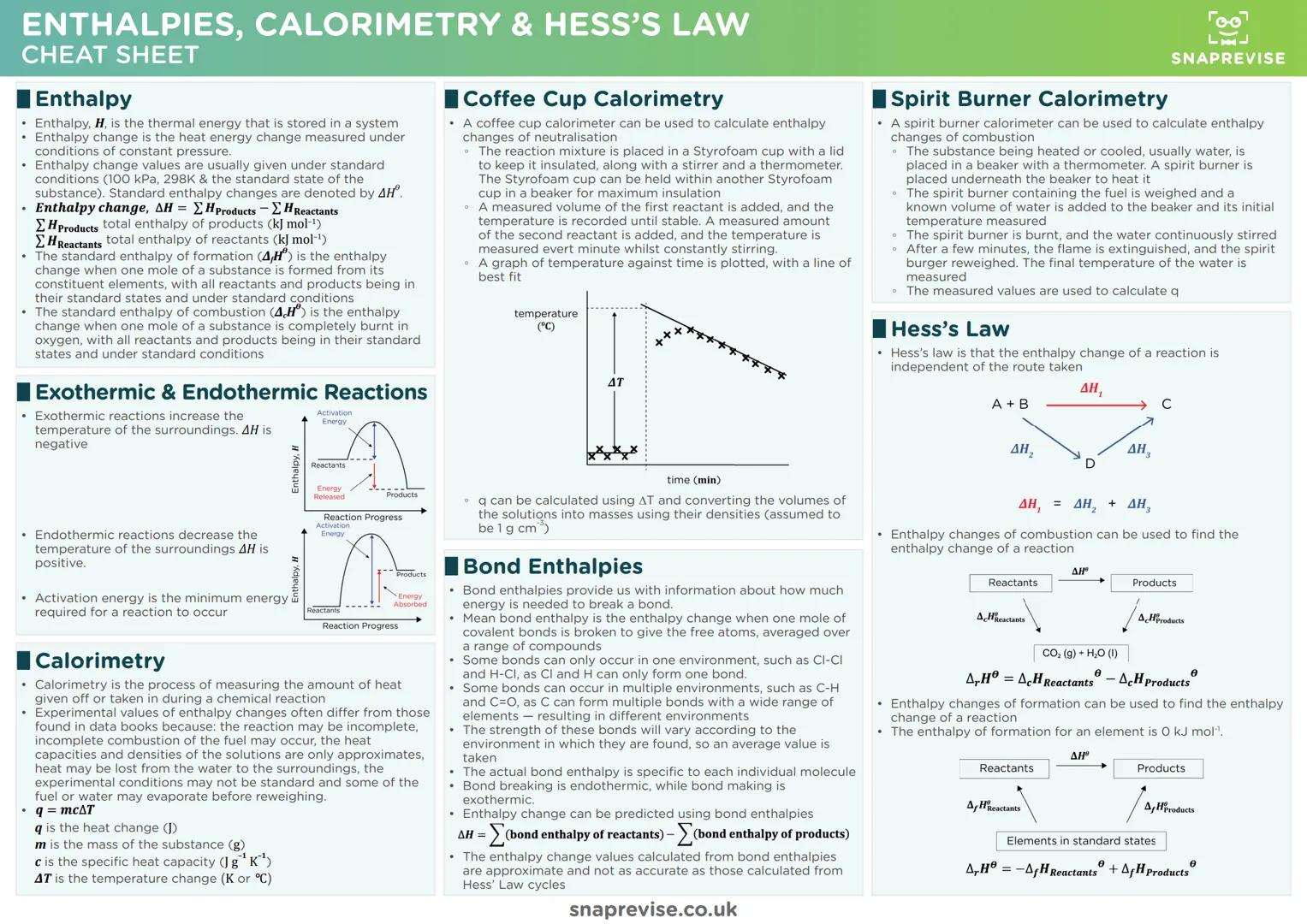
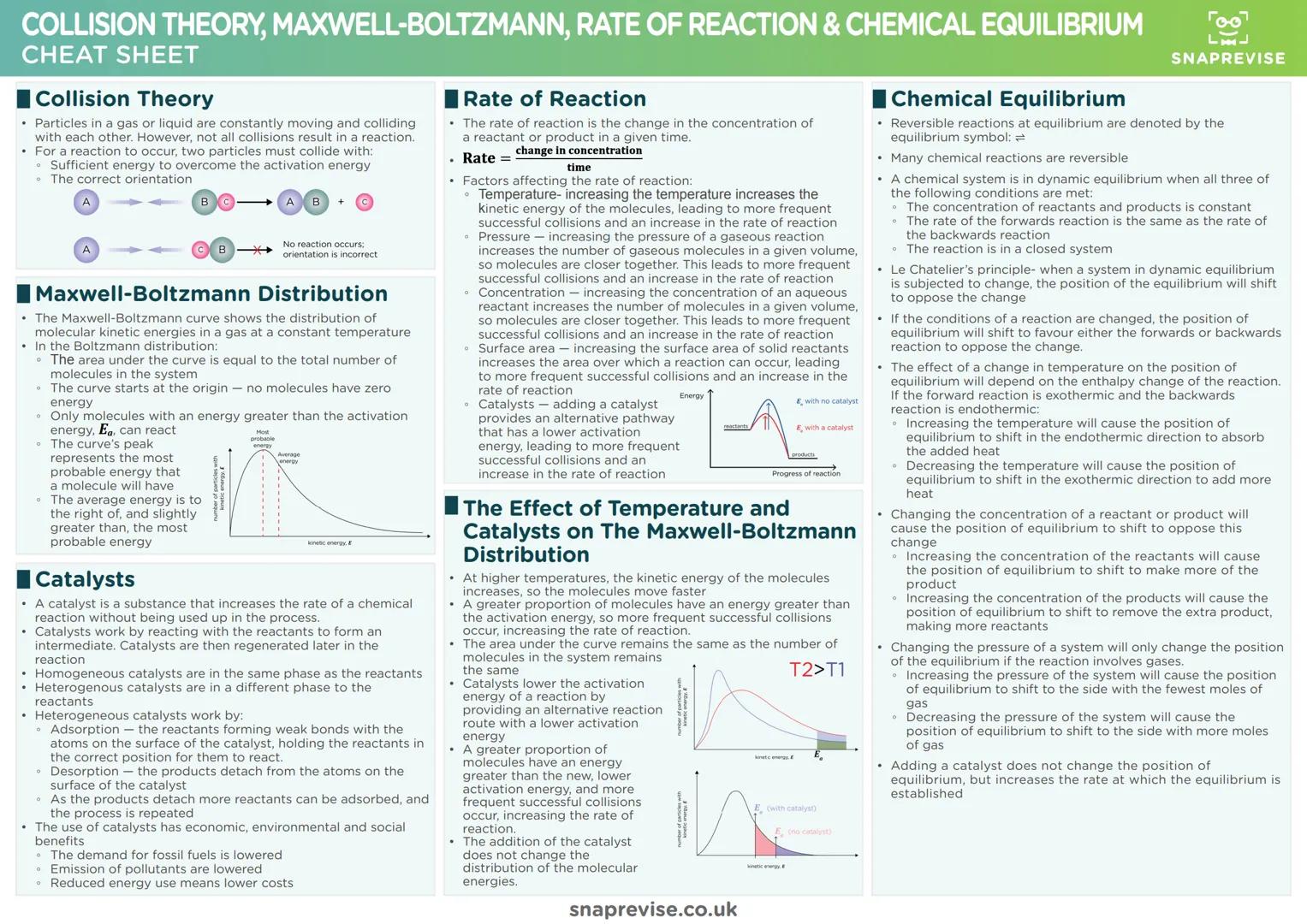
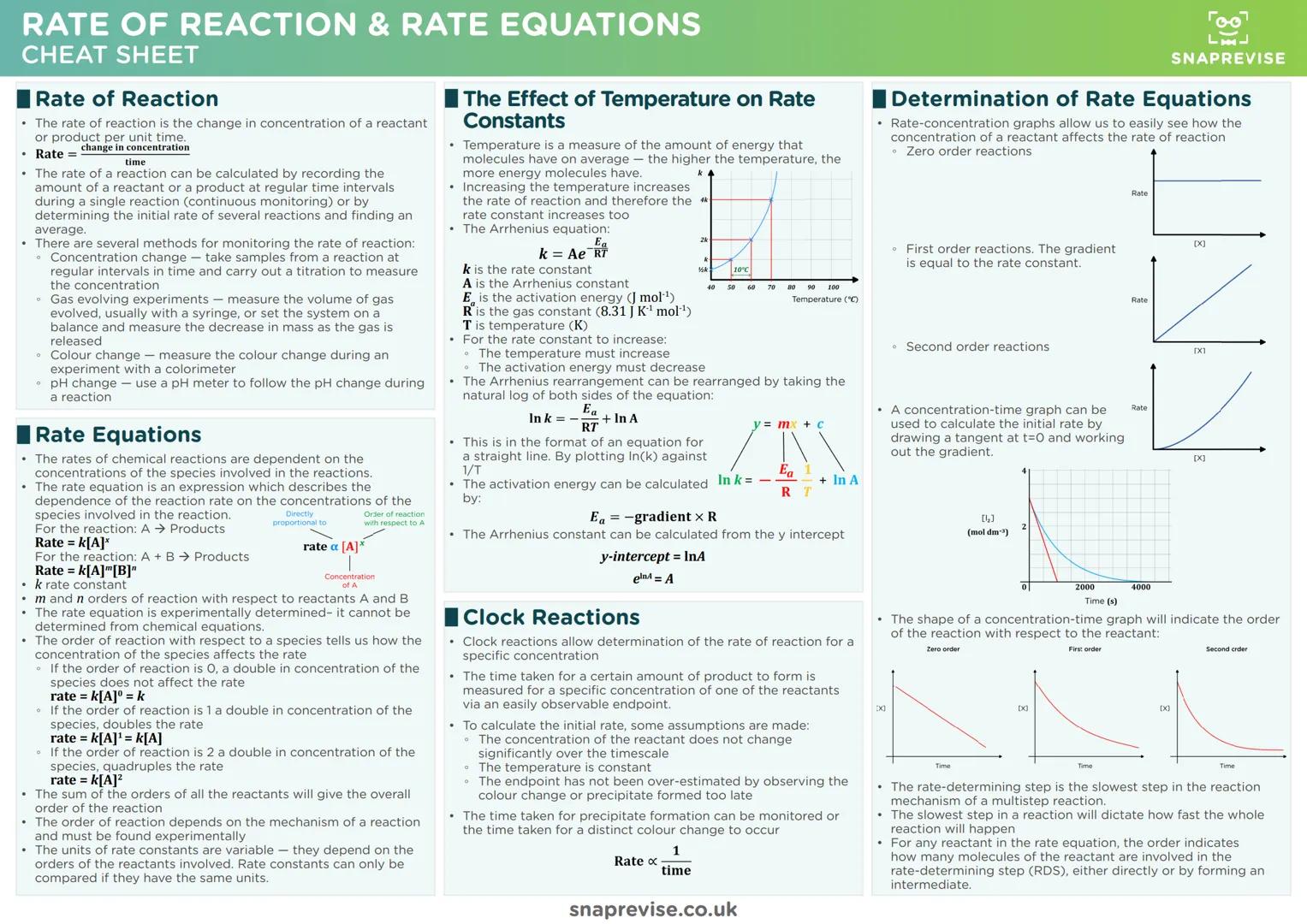
Chemical Bonding
Ionic bonding occurs when metals lose electrons and non-metals gain them, creating charged ions that attract electrostatically. The strength depends on charge highercharges=strongerbonds and size smallerions=strongerbondsduetocloserpacking.
Covalent bonding happens when non-metals share electrons to get full outer shells. You can have single, double, or triple bonds depending on how many pairs are shared. Coordinate bonds are special - one atom donates both electrons in the pair.
Molecular shapes are determined by electron pair repulsion. Bonding pairs and lone pairs repel each other, but lone pairs repel more strongly, reducing bond angles by about 2.5° each. Learn the common shapes: linear (180°), trigonal planar (120°), tetrahedral (109.5°).
Metallic bonding creates a "sea" of delocalised electrons around positive metal ions. This explains why metals conduct electricity, are malleable, and have high melting points. Stronger metallic bonding occurs with smaller, more highly charged metal ions.
Different crystal structures have characteristic properties - ionic compounds conduct when molten, metals conduct in all states, and molecular substances like ice have low melting points.
Memory Aid: "Lone pairs are bullies" - they push bonding pairs around more than other bonding pairs do!