Chemical reactions are happening all around you every day, from... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
1,646
•
13 Feb 2026
•
Stefany
@stef_mvp
Chemical reactions are happening all around you every day, from... Show more






Ever wondered why some reactions happen instantly whilst others take ages? The rate of reaction tells you exactly how fast a chemical change occurs. You can measure it by tracking either how quickly products form or how fast reactants disappear over time.
Collision theory explains why reactions happen at all. Particles must crash into each other with enough energy to actually react - this minimum energy needed is called the activation energy. Think of it like trying to break through a brick wall - you need enough force to make it happen.
Most reactions start fast because there are loads of particles bumping into each other. As the reaction continues, fewer reactant particles remain, so collisions become less frequent and the reaction slows down. Eventually, one reactant runs out completely and the reaction stops.
Quick Tip: Remember that particles need both collision AND sufficient energy to react successfully.

One clever way to track reaction speed involves watching mass disappear as gas escapes. When calcium carbonate reacts with hydrochloric acid, carbon dioxide gas bubbles away, making the reaction mixture lighter over time.
You'll place the whole setup on a balance and record the mass every few seconds. Cotton wool stops acid from spitting out (safety first!), but still lets gas escape. The faster the mass decreases, the quicker your reaction is happening.
This method works brilliantly because digital balances are incredibly accurate. However, there's one downside - the gas escapes straight into the air around you, which isn't ideal in a classroom setting.
Exam Hint: Always explain that mass decreases because gas escapes, not because matter disappears!
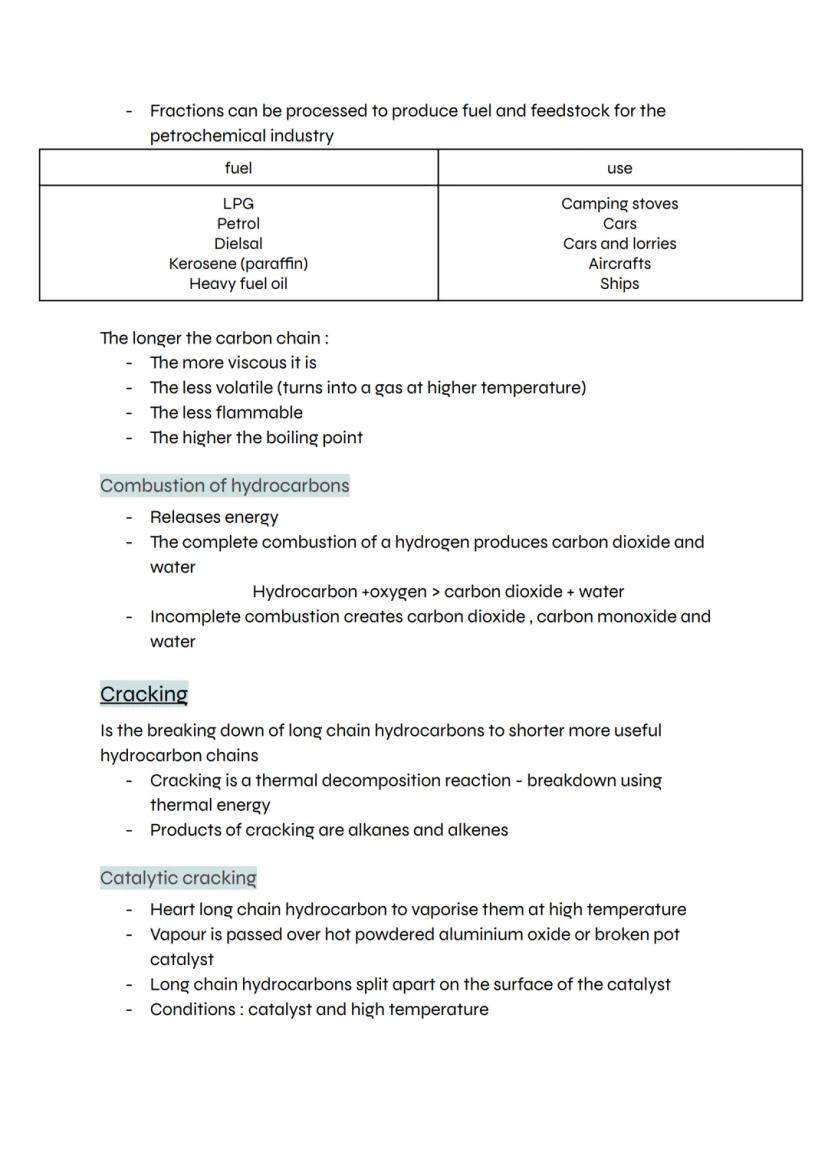
Gas syringes give you a more precise way to measure reaction rates by directly capturing the gas produced. When magnesium reacts with hydrochloric acid, hydrogen gas pushes the syringe plunger out, showing you exactly how much gas forms.
The method is straightforward: weigh your solid reactant, add it to acid in a flask, then connect the gas syringe and start timing. Record the gas volume every 10 seconds to track the reaction's progress.
Gas syringes beat measuring cylinders for accuracy because they have much better resolution - you can read smaller volume changes. Just watch out for vigorous reactions that might blow the plunger right off!
Safety Note: Always ensure the gas syringe is properly connected before starting the reaction.
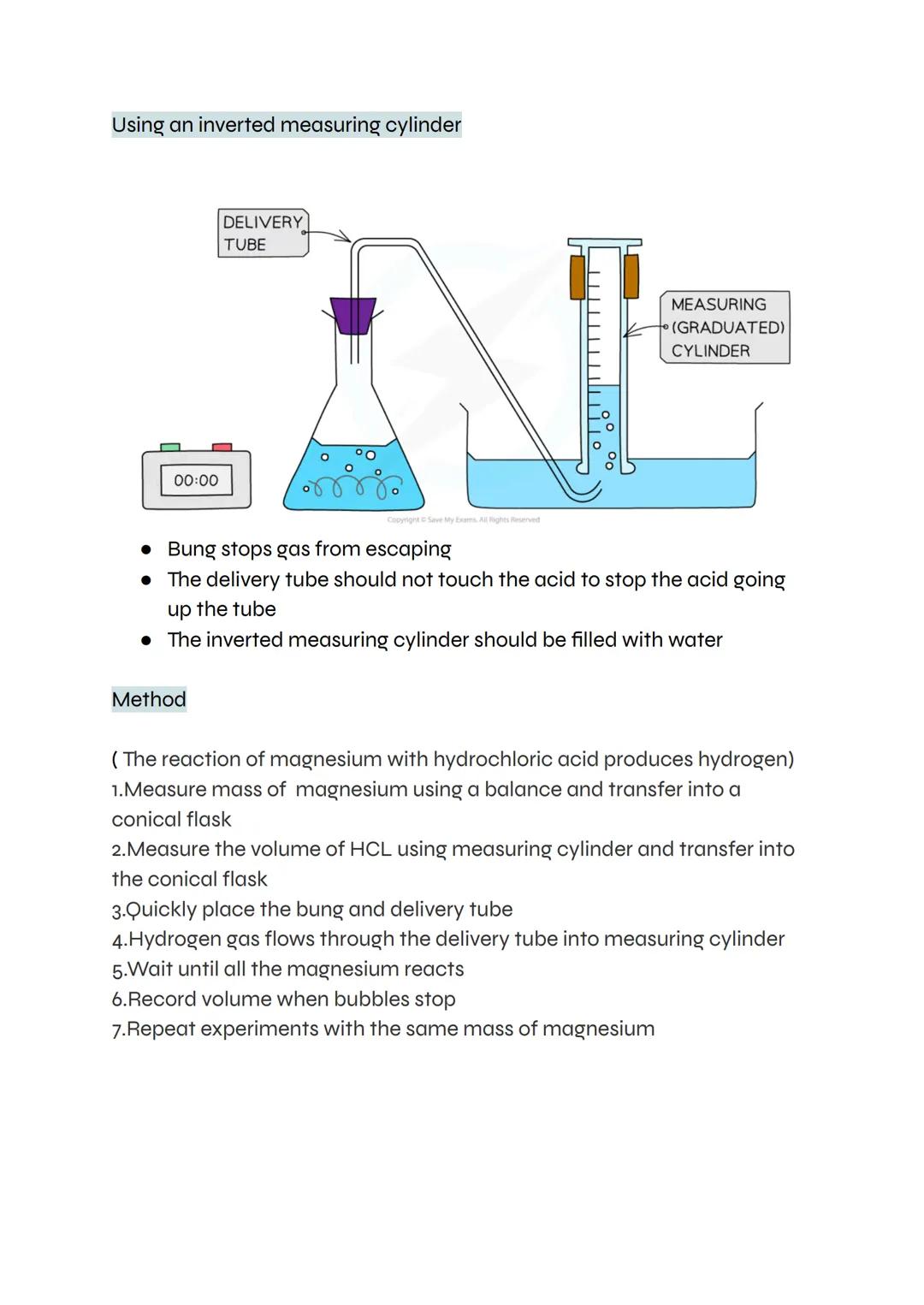
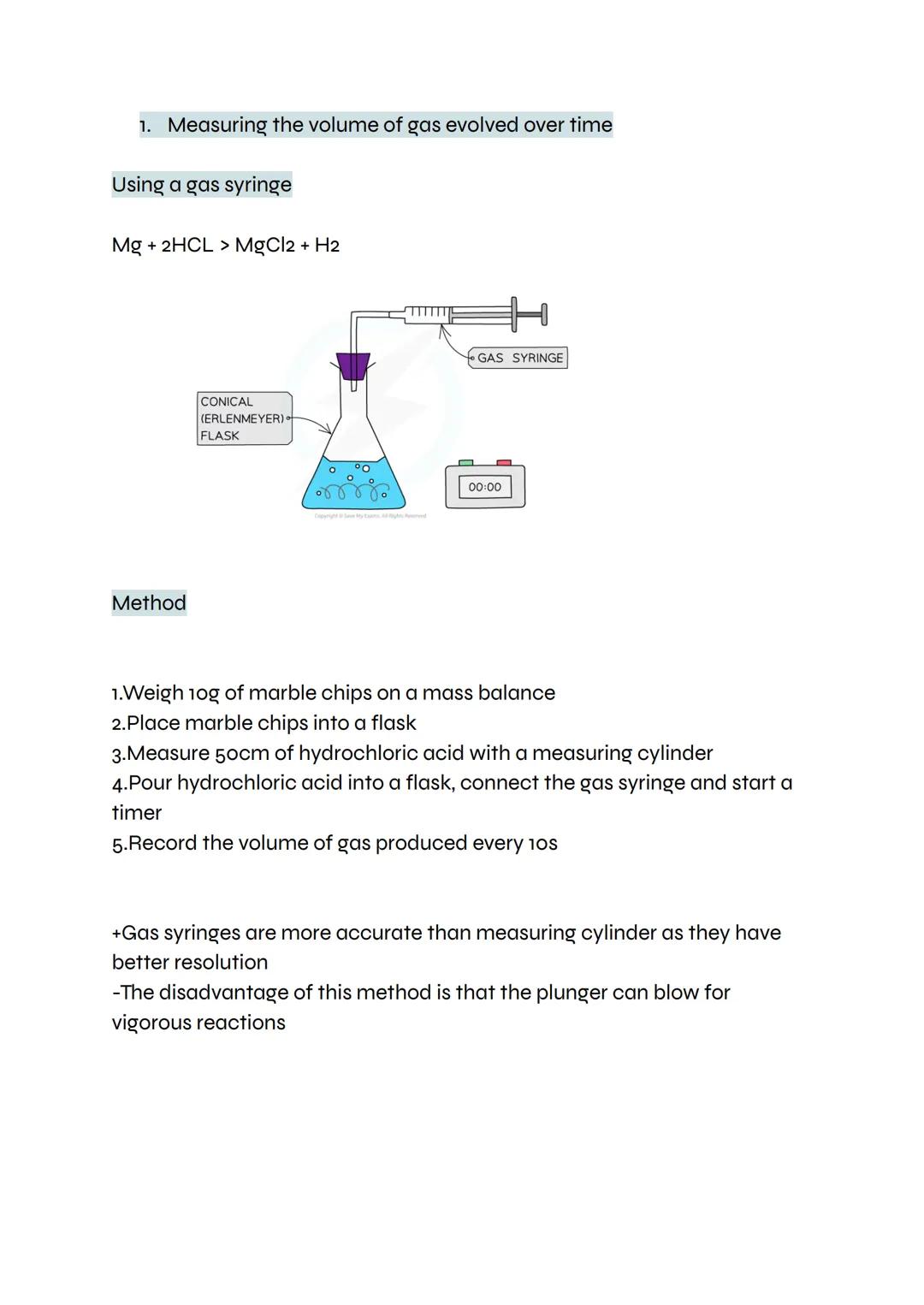
This classic method uses an upside-down measuring cylinder filled with water to collect gas. As hydrogen bubbles through the delivery tube, it displaces water and you can measure exactly how much gas forms.
The setup needs careful attention to detail. The bung must seal tightly to prevent gas escaping, and the delivery tube shouldn't touch the acid (otherwise acid might travel up the tube). Once everything's connected, wait until bubbling stops completely.
This technique works well for reactions that produce a steady stream of gas. You can repeat the experiment multiple times with the same mass of magnesium to check your results are reliable.
Top Tip: Fill the measuring cylinder completely with water before inverting it - any air bubbles will mess up your measurements.
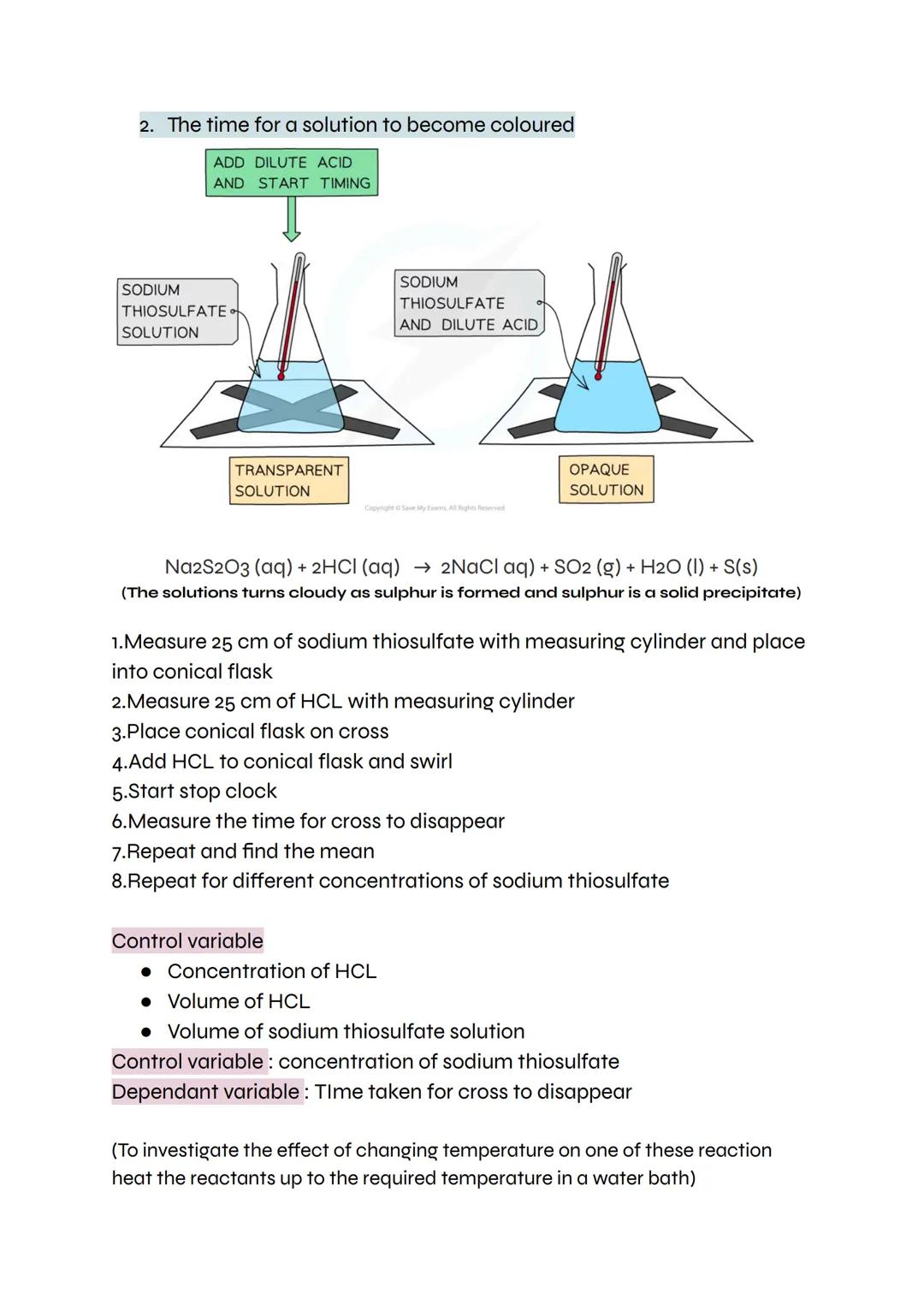
Some reactions create products that change how clearly you can see through a solution. When sodium thiosulfate reacts with hydrochloric acid, solid sulfur forms, making the mixture increasingly cloudy until you can't see through it.
Place a cross under your reaction flask and time how long it takes to disappear completely. This method works perfectly for investigating how concentration affects reaction rate - just change the concentration of sodium thiosulfate whilst keeping everything else constant.
Your control variables include the concentration and volume of hydrochloric acid, plus the volume of sodium thiosulfate solution. The dependent variable is the time taken for the cross to vanish. Want to investigate temperature effects? Just heat your reactants in a water bath first.
Remember: The faster the cross disappears, the quicker the reaction rate!
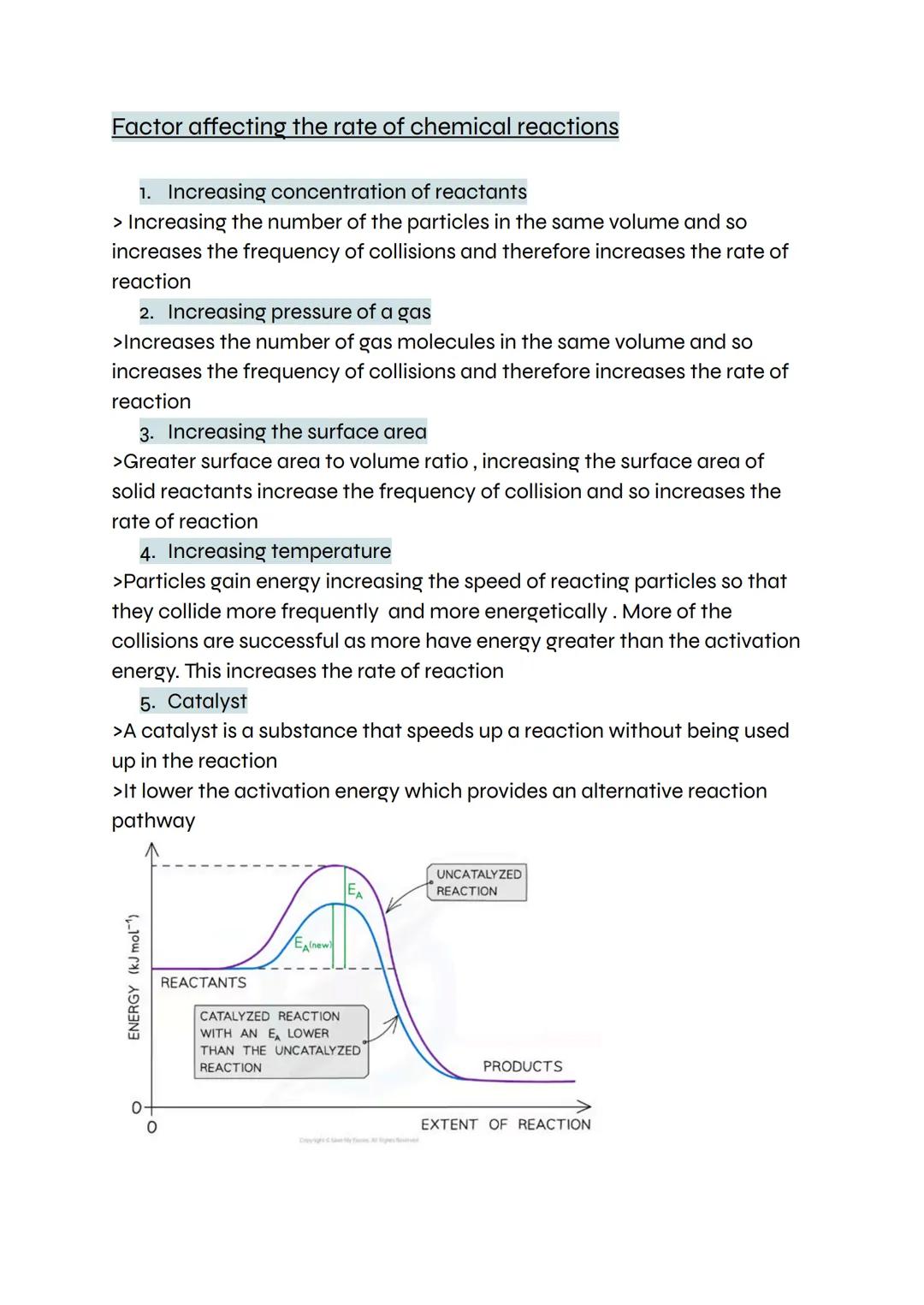
Five main factors control how fast chemical reactions happen, and understanding them helps you predict and control reaction speeds. Concentration matters because more particles in the same space means more frequent collisions and faster reactions.
Pressure works similarly for gases - squashing gas molecules together increases collision frequency. Surface area is crucial for solid reactants because breaking them into smaller pieces exposes more surface for reactions to occur on.
Temperature has the biggest impact because it makes particles move faster and hit each other harder. More collisions happen, and more of them have enough energy to overcome the activation energy. Catalysts speed things up by providing an alternative pathway with lower activation energy.
Exam Focus: Learn to explain each factor using collision theory - examiners love this connection!

Some reactions can go both ways - products can turn back into reactants under the right conditions. Dynamic equilibrium occurs when the forward and reverse reactions happen at exactly the same rate in a closed system.
A brilliant example is copper sulfate changing between its hydrated (blue) and anhydrous (white) forms. Heat drives water off (blue to white), whilst adding water reverses this (white to blue). If a reaction is exothermic in one direction, it's always endothermic in the reverse direction.
Ammonium chloride shows this beautifully - heating the white solid produces colourless gases, but cooling makes them recombine into the white solid again. The key is that equilibrium only happens when nothing can escape from your reaction vessel.
Key Point: Dynamic equilibrium means reactions are still happening - they're just balanced perfectly!

Le Chatelier's Principle predicts how equilibrium systems respond to changes - they always try to counteract whatever you do to them. It's like a chemical balancing act that automatically adjusts to maintain stability.
Temperature changes shift equilibrium towards the endothermic direction when heated, or the exothermic direction when cooled. Pressure changes in gas reactions favour the side with fewer molecules when increased, or more molecules when decreased.
Concentration changes trigger the system to use up excess reactants or make more products to restore balance. Interestingly, catalysts don't shift equilibrium position at all - they speed up both forward and reverse reactions equally.
Memory Trick: Think of equilibrium as a stubborn system that always fights back against changes!
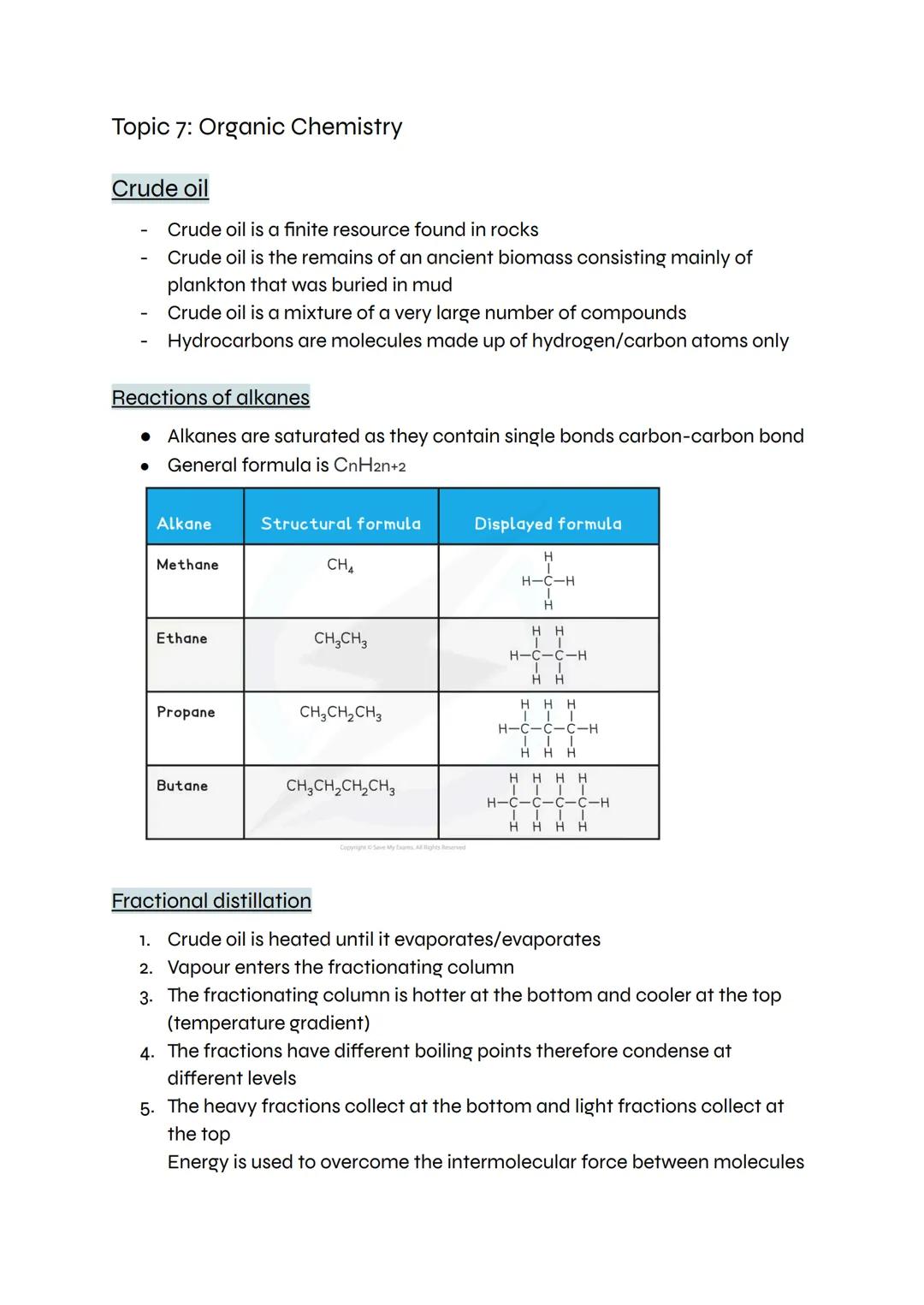
Crude oil formed millions of years ago from ancient plankton buried in mud - it's basically fossilised sea life! This finite resource contains thousands of different hydrocarbons (molecules made only of hydrogen and carbon atoms).
Alkanes are the simplest hydrocarbons with the general formula CnH2n+2. They're saturated because all carbon atoms are connected by single bonds. You need to know methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10).
Fractional distillation separates crude oil by heating it until everything evaporates, then cooling different fractions at different temperatures. Heavy molecules condense first (bottom of the column), whilst light molecules rise higher before condensing. This works because different sized molecules have different boiling points.
Quick Fact: Longer hydrocarbon chains are more viscous, less volatile, less flammable, and have higher boiling points!
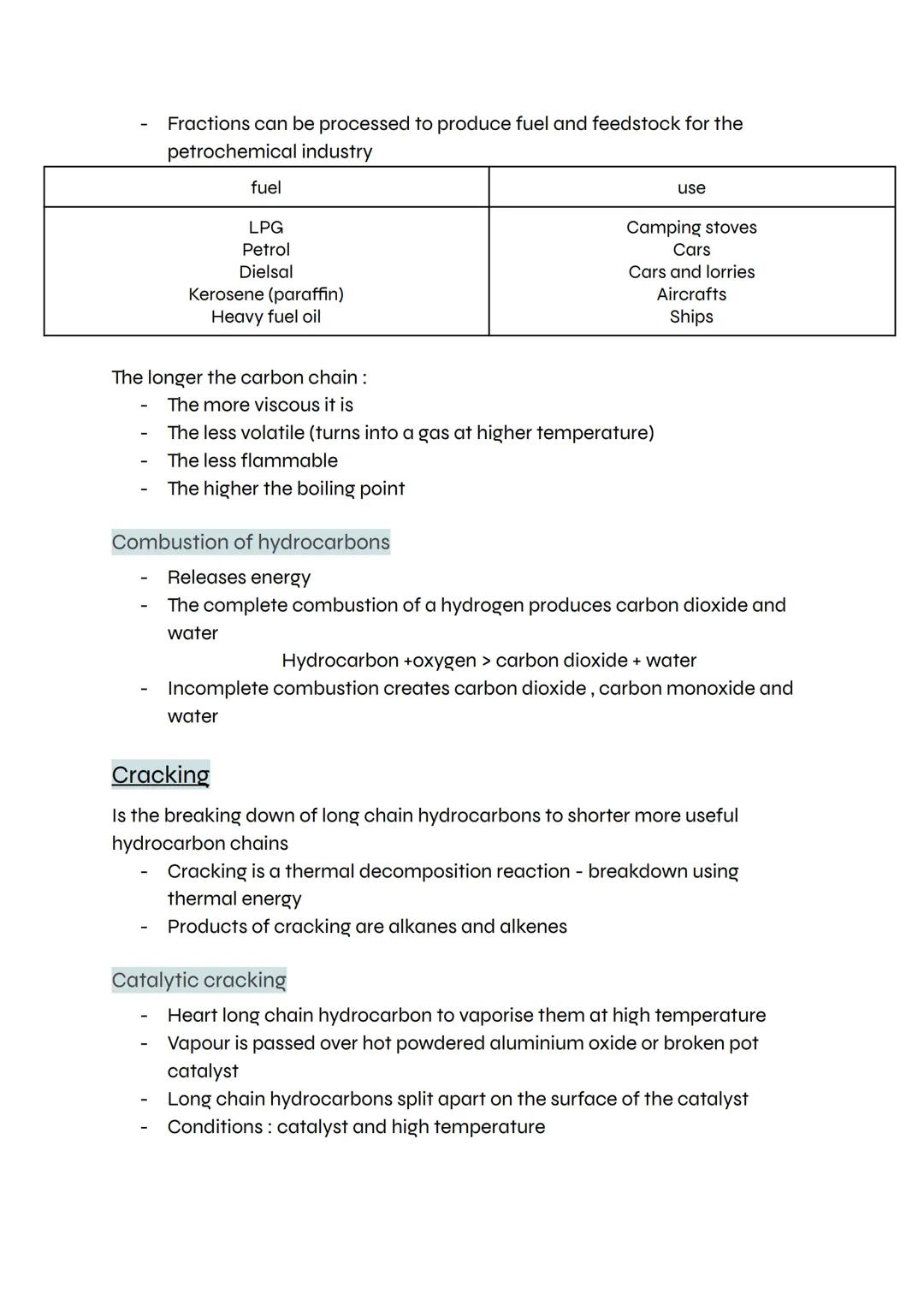
Different fractions from crude oil become fuels for different purposes - LPG for camping stoves, petrol for cars, diesel for lorries, kerosene for aircraft, and heavy fuel oil for ships. Each fraction has properties perfectly suited to its use.
Combustion of hydrocarbons releases energy that powers our world. Complete combustion produces carbon dioxide and water, but incomplete combustion also creates dangerous carbon monoxide. The equation is simple: hydrocarbon + oxygen → carbon dioxide + water.
Cracking breaks down long, less useful hydrocarbon chains into shorter, more valuable ones. This thermal decomposition reaction uses high temperatures and catalysts (like aluminium oxide) to split large molecules. The products include both alkanes and alkenes, giving us more useful fuels and chemicals.
Industrial Importance: Cracking helps match supply with demand - we need more petrol than crude oil naturally provides!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Stefany
@stef_mvp
Chemical reactions are happening all around you every day, from the fuel in cars to the food you digest. Understanding how fast these reactions happen and what affects their speed is crucial for everything from industrial processes to your GCSE... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered why some reactions happen instantly whilst others take ages? The rate of reaction tells you exactly how fast a chemical change occurs. You can measure it by tracking either how quickly products form or how fast reactants disappear over time.
Collision theory explains why reactions happen at all. Particles must crash into each other with enough energy to actually react - this minimum energy needed is called the activation energy. Think of it like trying to break through a brick wall - you need enough force to make it happen.
Most reactions start fast because there are loads of particles bumping into each other. As the reaction continues, fewer reactant particles remain, so collisions become less frequent and the reaction slows down. Eventually, one reactant runs out completely and the reaction stops.
Quick Tip: Remember that particles need both collision AND sufficient energy to react successfully.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
One clever way to track reaction speed involves watching mass disappear as gas escapes. When calcium carbonate reacts with hydrochloric acid, carbon dioxide gas bubbles away, making the reaction mixture lighter over time.
You'll place the whole setup on a balance and record the mass every few seconds. Cotton wool stops acid from spitting out (safety first!), but still lets gas escape. The faster the mass decreases, the quicker your reaction is happening.
This method works brilliantly because digital balances are incredibly accurate. However, there's one downside - the gas escapes straight into the air around you, which isn't ideal in a classroom setting.
Exam Hint: Always explain that mass decreases because gas escapes, not because matter disappears!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
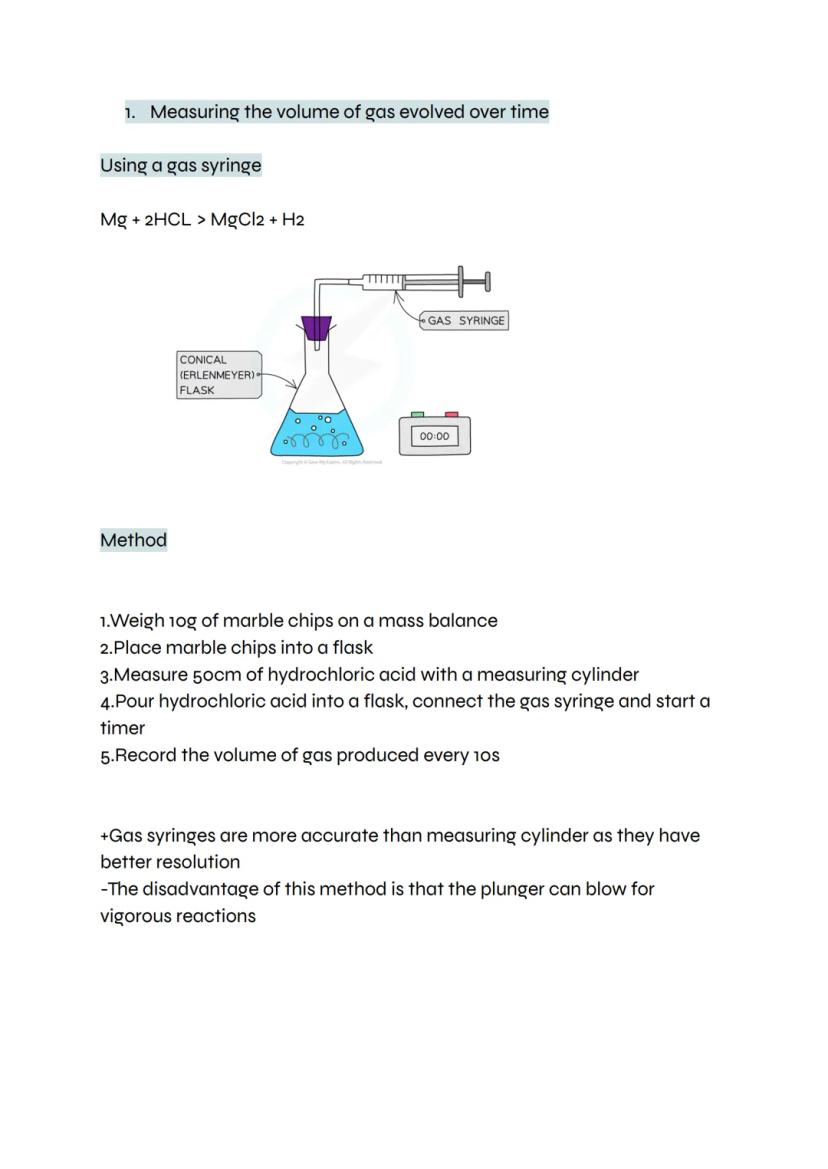
Gas syringes give you a more precise way to measure reaction rates by directly capturing the gas produced. When magnesium reacts with hydrochloric acid, hydrogen gas pushes the syringe plunger out, showing you exactly how much gas forms.
The method is straightforward: weigh your solid reactant, add it to acid in a flask, then connect the gas syringe and start timing. Record the gas volume every 10 seconds to track the reaction's progress.
Gas syringes beat measuring cylinders for accuracy because they have much better resolution - you can read smaller volume changes. Just watch out for vigorous reactions that might blow the plunger right off!
Safety Note: Always ensure the gas syringe is properly connected before starting the reaction.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
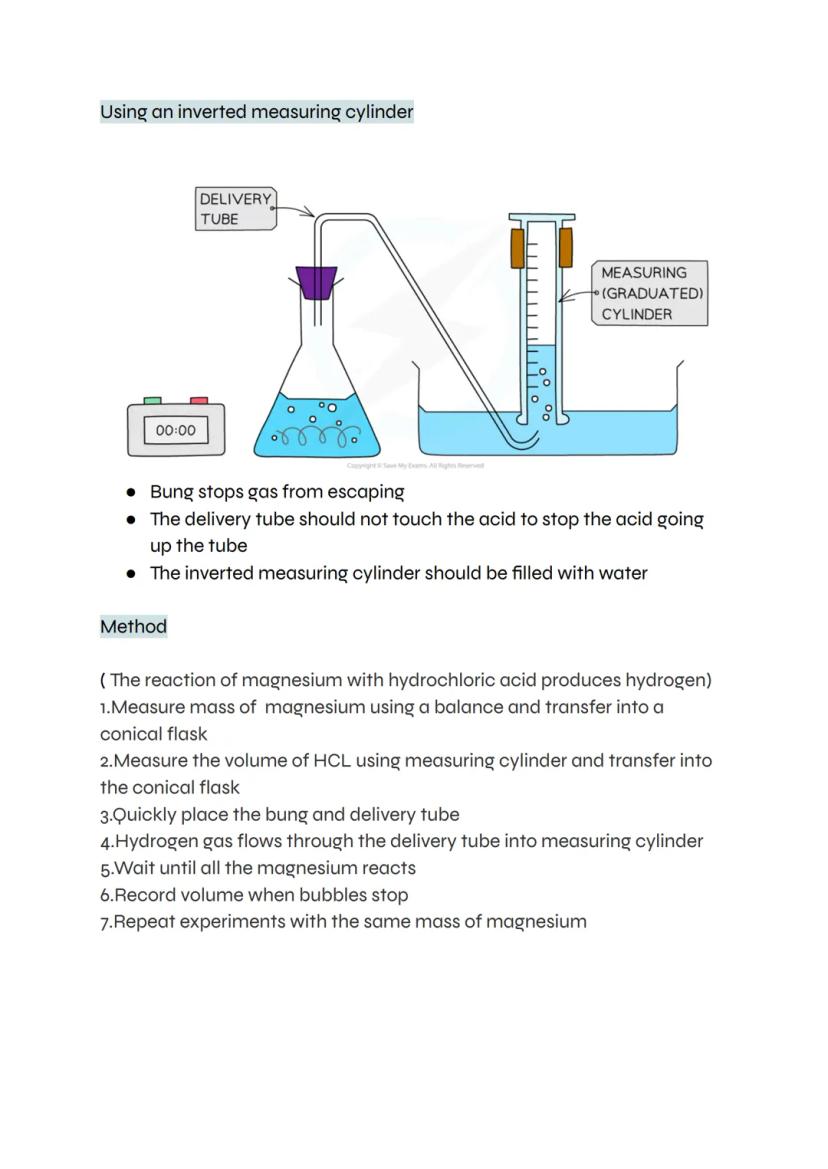
This classic method uses an upside-down measuring cylinder filled with water to collect gas. As hydrogen bubbles through the delivery tube, it displaces water and you can measure exactly how much gas forms.
The setup needs careful attention to detail. The bung must seal tightly to prevent gas escaping, and the delivery tube shouldn't touch the acid (otherwise acid might travel up the tube). Once everything's connected, wait until bubbling stops completely.
This technique works well for reactions that produce a steady stream of gas. You can repeat the experiment multiple times with the same mass of magnesium to check your results are reliable.
Top Tip: Fill the measuring cylinder completely with water before inverting it - any air bubbles will mess up your measurements.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
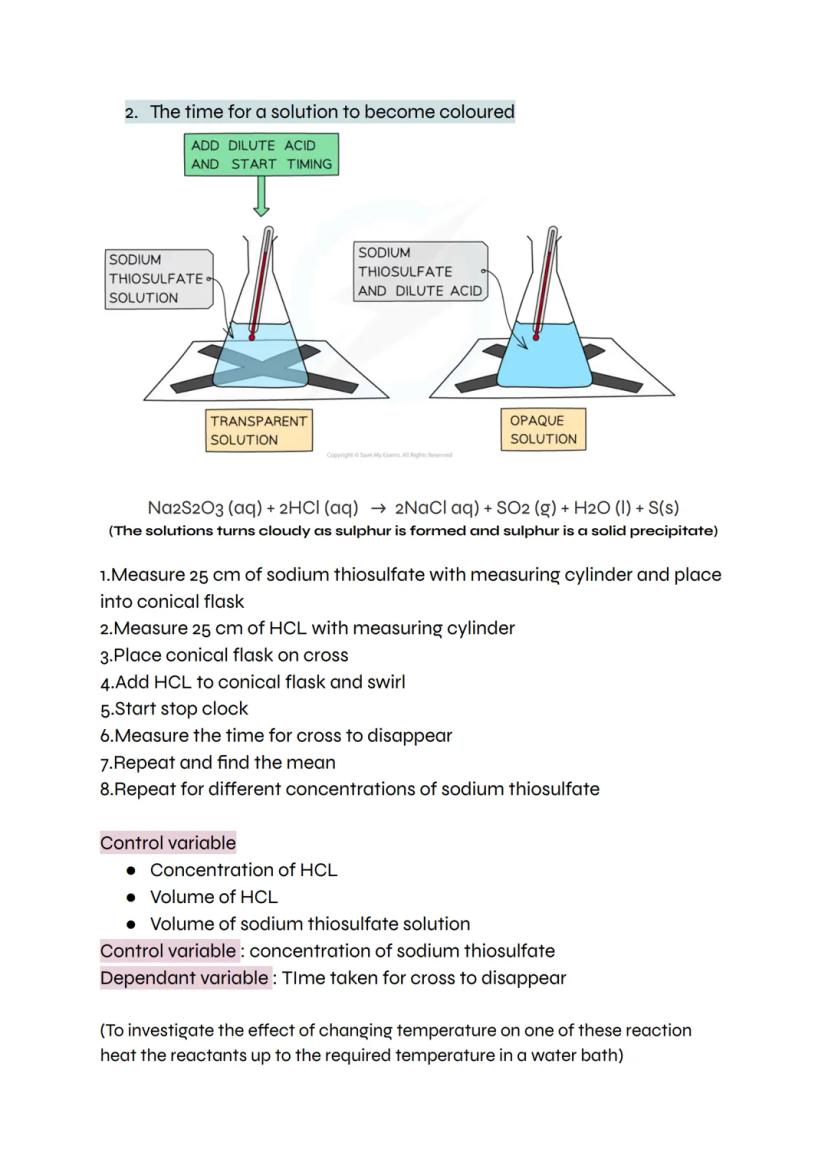
Some reactions create products that change how clearly you can see through a solution. When sodium thiosulfate reacts with hydrochloric acid, solid sulfur forms, making the mixture increasingly cloudy until you can't see through it.
Place a cross under your reaction flask and time how long it takes to disappear completely. This method works perfectly for investigating how concentration affects reaction rate - just change the concentration of sodium thiosulfate whilst keeping everything else constant.
Your control variables include the concentration and volume of hydrochloric acid, plus the volume of sodium thiosulfate solution. The dependent variable is the time taken for the cross to vanish. Want to investigate temperature effects? Just heat your reactants in a water bath first.
Remember: The faster the cross disappears, the quicker the reaction rate!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
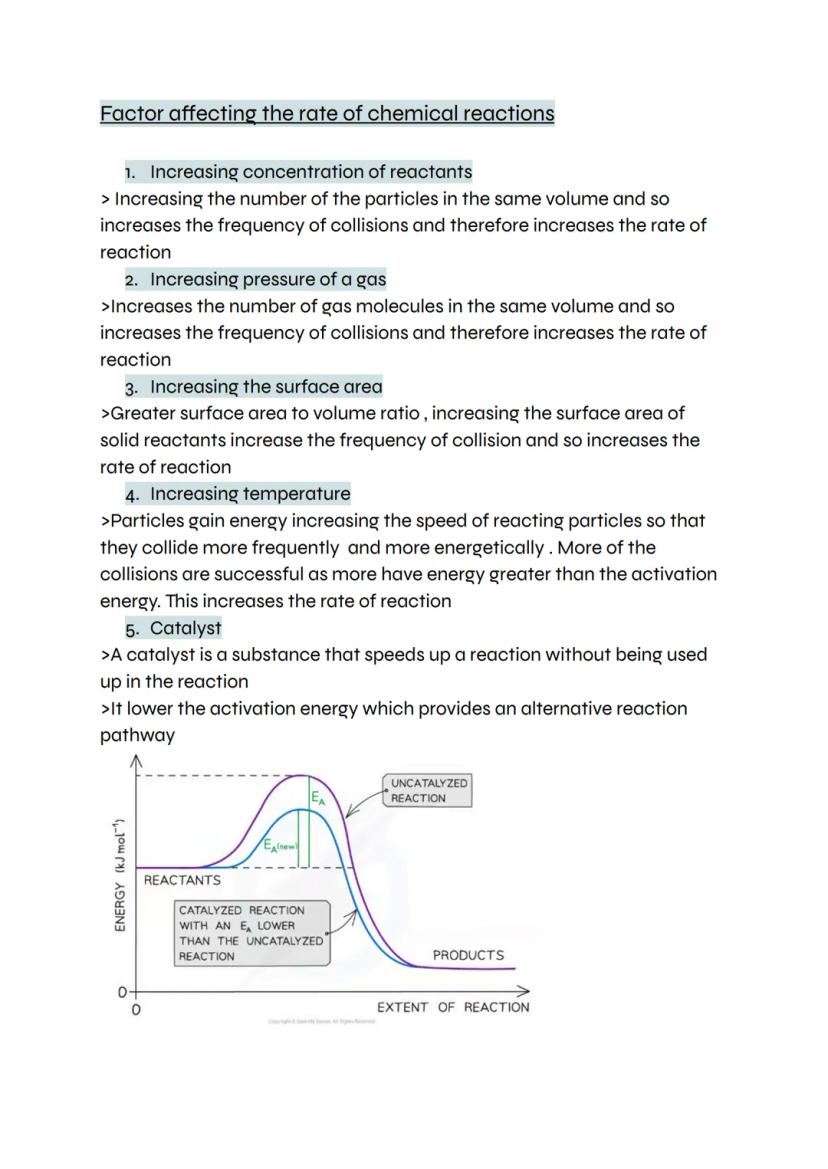
Five main factors control how fast chemical reactions happen, and understanding them helps you predict and control reaction speeds. Concentration matters because more particles in the same space means more frequent collisions and faster reactions.
Pressure works similarly for gases - squashing gas molecules together increases collision frequency. Surface area is crucial for solid reactants because breaking them into smaller pieces exposes more surface for reactions to occur on.
Temperature has the biggest impact because it makes particles move faster and hit each other harder. More collisions happen, and more of them have enough energy to overcome the activation energy. Catalysts speed things up by providing an alternative pathway with lower activation energy.
Exam Focus: Learn to explain each factor using collision theory - examiners love this connection!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Some reactions can go both ways - products can turn back into reactants under the right conditions. Dynamic equilibrium occurs when the forward and reverse reactions happen at exactly the same rate in a closed system.
A brilliant example is copper sulfate changing between its hydrated (blue) and anhydrous (white) forms. Heat drives water off (blue to white), whilst adding water reverses this (white to blue). If a reaction is exothermic in one direction, it's always endothermic in the reverse direction.
Ammonium chloride shows this beautifully - heating the white solid produces colourless gases, but cooling makes them recombine into the white solid again. The key is that equilibrium only happens when nothing can escape from your reaction vessel.
Key Point: Dynamic equilibrium means reactions are still happening - they're just balanced perfectly!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Le Chatelier's Principle predicts how equilibrium systems respond to changes - they always try to counteract whatever you do to them. It's like a chemical balancing act that automatically adjusts to maintain stability.
Temperature changes shift equilibrium towards the endothermic direction when heated, or the exothermic direction when cooled. Pressure changes in gas reactions favour the side with fewer molecules when increased, or more molecules when decreased.
Concentration changes trigger the system to use up excess reactants or make more products to restore balance. Interestingly, catalysts don't shift equilibrium position at all - they speed up both forward and reverse reactions equally.
Memory Trick: Think of equilibrium as a stubborn system that always fights back against changes!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Crude oil formed millions of years ago from ancient plankton buried in mud - it's basically fossilised sea life! This finite resource contains thousands of different hydrocarbons (molecules made only of hydrogen and carbon atoms).
Alkanes are the simplest hydrocarbons with the general formula CnH2n+2. They're saturated because all carbon atoms are connected by single bonds. You need to know methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10).
Fractional distillation separates crude oil by heating it until everything evaporates, then cooling different fractions at different temperatures. Heavy molecules condense first (bottom of the column), whilst light molecules rise higher before condensing. This works because different sized molecules have different boiling points.
Quick Fact: Longer hydrocarbon chains are more viscous, less volatile, less flammable, and have higher boiling points!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Different fractions from crude oil become fuels for different purposes - LPG for camping stoves, petrol for cars, diesel for lorries, kerosene for aircraft, and heavy fuel oil for ships. Each fraction has properties perfectly suited to its use.
Combustion of hydrocarbons releases energy that powers our world. Complete combustion produces carbon dioxide and water, but incomplete combustion also creates dangerous carbon monoxide. The equation is simple: hydrocarbon + oxygen → carbon dioxide + water.
Cracking breaks down long, less useful hydrocarbon chains into shorter, more valuable ones. This thermal decomposition reaction uses high temperatures and catalysts (like aluminium oxide) to split large molecules. The products include both alkanes and alkenes, giving us more useful fuels and chemicals.
Industrial Importance: Cracking helps match supply with demand - we need more petrol than crude oil naturally provides!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
51
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the fundamentals of chemical reactions, including the roles of reactants and products, and learn how to write and balance word and symbol equations. This summary covers key concepts such as measurable energy changes and the conservation of mass in reactions.
Learn the Bubble Method for balancing chemical equations effectively. This guide provides a step-by-step approach to ensure equal numbers of each element on both sides of the equation, focusing on sodium and oxygen in examples. Ideal for chemistry students looking to master equation balancing techniques.
Explore the principles of chemical reaction kinetics, including the factors affecting reaction rates, the concept of equilibrium in reversible reactions, and the role of temperature, pressure, and concentration. This summary covers key concepts such as La Chatelier's principle, collision theory, and the impact of catalysts on reaction speed. Ideal for AQA Chemistry Paper 2, C6.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user