Analytical chemistry is the detective work of the scientific world... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
215
•
16 Feb 2026
•
rafallaith
@rafallaith_9djz3m5vk
Analytical chemistry is the detective work of the scientific world... Show more









This is your introduction to analytical chemistry, taught by Dr. Jwan Oday Abdulsattar. You'll be exploring the fundamental concepts that help scientists identify and measure chemical compounds in everything from medical samples to crime scene evidence.
The course combines theoretical knowledge with practical mathematical problem-solving skills. You'll discover how analytical chemistry connects to real-world applications across medicine, forensics, and industry.
Quick Tip: Think of analytical chemistry as being like a detective - you're gathering clues to solve chemical mysteries!

Analytical chemistry is essentially the science of answering two crucial questions: "What's in this sample?" and "How much is there?" It involves three key processes: separation, identification, and quantification of chemical compounds.
The field splits into two main branches. Qualitative analysis tells you what's present in a sample - think of it as making a list of ingredients. Quantitative analysis measures exactly how much of each component is there - like getting precise measurements for a recipe.
This isn't just academic theory - analytical chemistry powers everything from diabetes testing to solving crimes. Doctors use it for medical diagnoses, forensic scientists analyse evidence, and engineers ensure product quality. Qualitative chemical analysis specifically focuses on identifying whether ions, atoms, or compounds are present or absent.
Before starting any analysis, you need to determine if your sample contains organic compounds or inorganic compounds . This classification determines which analytical methods you'll use.
Real-World Connection: Every time you take a home pregnancy test or check your blood sugar, you're using analytical chemistry principles!

You don't always need expensive equipment for qualitative analysis - sometimes your senses are enough! Simple identification can be based on observable properties like colour, odour, melting point, boiling point, texture, and electrical conductivity.
The most common approach involves adding chemical reagents to your sample and watching what happens. These reagents are chosen to react selectively with specific compounds, usually producing either a coloured complex or a precipitate that you can easily spot.
Think about a urine test - when you add the test reagent, colour changes indicate the presence of substances like glucose. High glucose levels suggest diabetes, but you'd need a quantitative blood test to get exact concentration values. Another classic example is testing for copper: when copper(II) reacts with ammonia, it forms a distinctive deep blue copper-ammonia complex.
The limitation of classical qualitative chemical analysis is that it usually only identifies parts of molecules rather than complete structures. That's why analysts often combine these tests with measurements of boiling points, melting points, and densities to get the full picture.
Study Hack: Remember that qualitative = quality (what type), not quantity (how much)!

Qualitative chemical analysis shines brightest in medical and forensic applications. Doctors rely on it for diagnostic tests - from checking blood chemistry to identifying infections. It's often the first step before more detailed quantitative testing.
In criminology, forensic scientists use these techniques to identify mysterious substances found at crime scenes. A simple colour test might reveal whether a white powder is cocaine or baking soda, potentially making or breaking a criminal case.
You've probably used qualitative analysis at home without realising it. When you check if margarine melts faster than butter, or test whether a cleaning product changes colour on different surfaces, you're doing basic qualitative analysis.
The beauty of this field is its immediate, practical nature. Unlike some areas of chemistry that seem purely theoretical, qualitative analysis gives you instant, visible results that have real-world consequences.
Career Insight: Qualitative analysis skills are essential for careers in medicine, forensic science, environmental monitoring, and quality control!

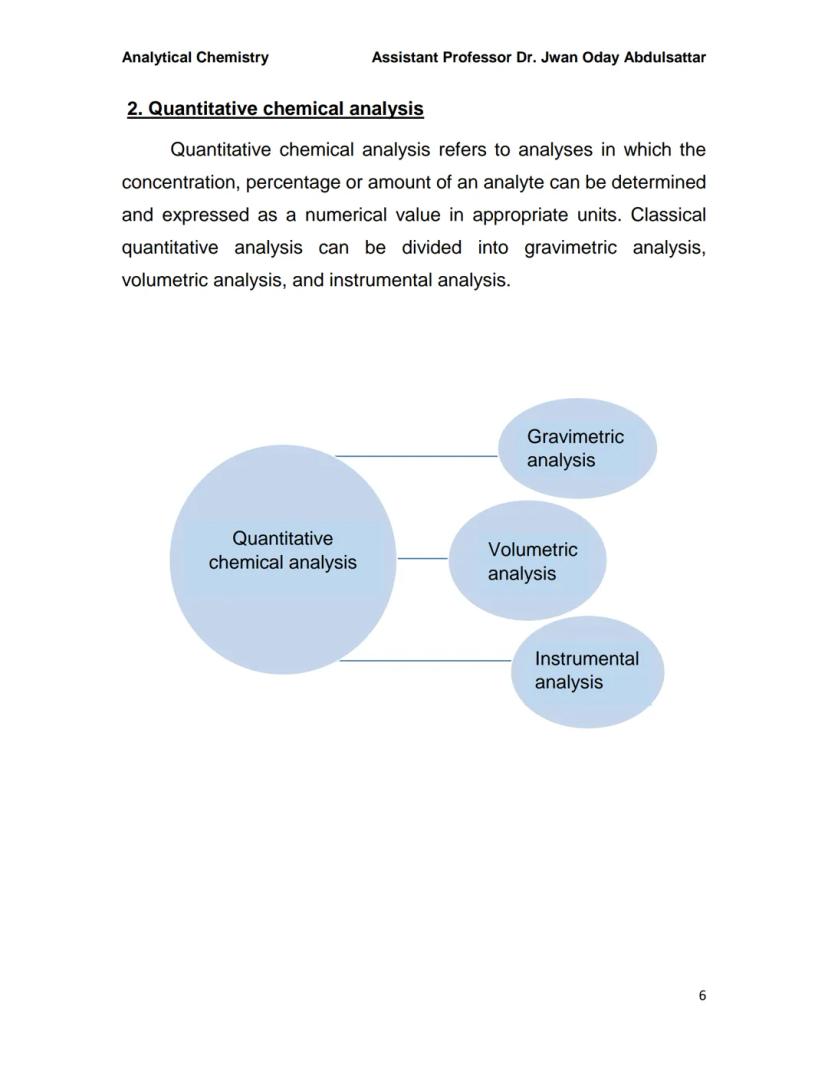
This page provides a visual overview of chemical analysis structure, showing how the field branches into qualitative and quantitative approaches.
Qualitative analysis forms the foundation - it's your starting point for understanding what you're dealing with before you can measure it accurately.
The diagram helps you visualise the systematic approach that analytical chemists use, moving from general identification to specific quantification based on what the situation requires.
Visual Learner Tip: Use this diagram as a mental map when tackling analytical chemistry problems!
Quantitative chemical analysis is where you get the numbers - determining exactly how much of a substance is present in your sample. Unlike qualitative analysis, this gives you measurable results expressed in units like grams, percentages, or concentrations.
The field divides into three main approaches: gravimetric analysis (measuring mass), volumetric analysis (measuring volume), and instrumental analysis (using specialised equipment).
Each method has its strengths depending on what you're measuring and how precise you need to be. Gravimetric analysis is incredibly accurate but time-consuming, while volumetric analysis is faster and still very reliable.
Understanding when to use each technique is crucial for any analytical chemist. Your choice depends on factors like the sample type, required accuracy, available time, and equipment.
Exam Tip: Learn the three types of quantitative analysis and when to use each one - this often appears in exam questions!
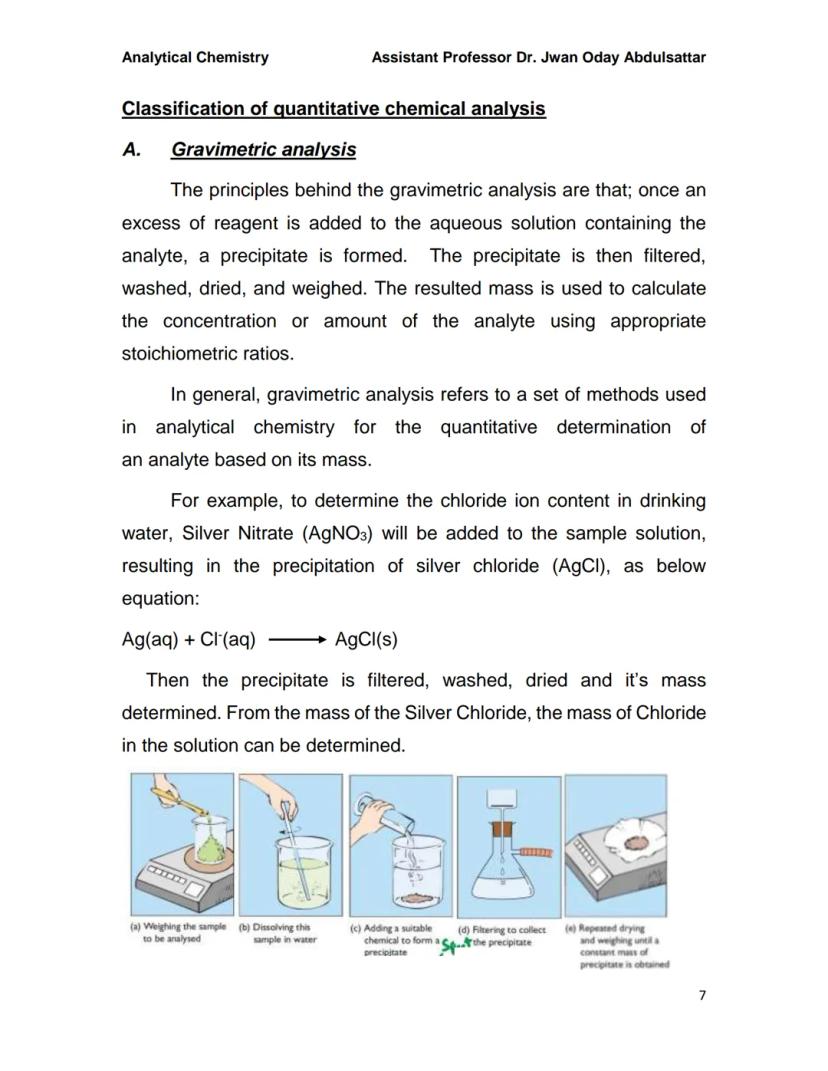
Gravimetric analysis is beautifully simple in principle: you add a reagent that makes your target substance form a precipitate, then you filter, wash, dry, and weigh it. The mass tells you exactly how much of your original substance was present.
Here's how it works: add excess reagent to your sample solution, causing precipitation. Filter out the solid, wash it thoroughly to remove impurities, dry it completely, then weigh it. Using stoichiometric ratios, you can calculate back to find your original concentration.
Take measuring chloride in drinking water as an example. Add silver nitrate (AgNO₃) and watch silver chloride (AgCl) precipitate out: Ag⁺(aq) + Cl⁻(aq) → AgCl(s). Weigh the AgCl precipitate, and you can calculate exactly how much chloride was in your water sample.
The process involves five clear steps: weighing your sample, dissolving it, adding precipitating reagent, filtering the precipitate, and repeated drying/weighing until you get a constant mass. This method is incredibly accurate but requires patience and careful technique.
Lab Success: Always dry your precipitate to constant mass - this means weighing, drying, and reweighing until the mass doesn't change!

Volumetric analysis (also called titrimetric analysis) is like a chemical balancing act. You gradually add a solution of known concentration (the titrant) to your unknown sample until you reach the equivalence point - where they're perfectly balanced chemically.
The key players are primary standard solutions and secondary standard solutions. Primary standards like sodium carbonate (Na₂CO₃) are ultra-pure reference materials that meet strict criteria: high purity, air stability, no water content, readily available, reasonably soluble, and large molar mass to minimise weighing errors.
Secondary standards are prepared in the lab and standardised against primary standards. Sodium hydroxide is a classic example - commercial NaOH contains impurities and absorbs water from air, so it can't be a primary standard.
The magic happens at the equivalence point, where moles of titrant equal moles of analyte. You'll often see this as a colour change, either natural or from an added indicator. The endpoint (where you see the colour change) approximates the equivalence point and lets you calculate your unknown concentration.
Practical Tip: The equivalence point is theoretical perfection; the endpoint is what you actually observe - they're close but not identical!

Biological titration shows how versatile volumetric analysis can be. In vaccine production, scientists use serial dilutions (1:1, 1:2, 1:4, 1:8) to count viruses and determine the titer - the dilution limit where viruses are still detectable.
Understanding solutions is fundamental to all this work. A solution is simply a homogeneous mixture where the solute (dissolved substance) disperses evenly throughout the solvent (the dissolving medium). Think salt (solute) dissolving in water (solvent) to make salt water.
The process is straightforward: NaCl(s) + H₂O(l) → Salt solution. The solid salt breaks apart and spreads uniformly throughout the water, creating a solution where every drop contains the same concentration.
These concepts underpin every quantitative analysis you'll perform. Whether you're measuring medicine concentrations or testing water purity, you're working with solutions and applying these same principles.
Memory Aid: Solute = dissolved (like sugar), Solvent = dissolves (like water), Solution = the final mixture!

Solutions behave differently depending on how much solute they contain. Unsaturated solutions have room for more solute - everything dissolves completely with no leftovers. It's like a car park with plenty of empty spaces.
Saturated solutions contain the maximum possible amount of dissolved solute at that temperature. Add more solute and it simply won't dissolve - it'll sit at the bottom like cars circling a full car park. This represents the natural solubility limit.
Supersaturated solutions are unstable chemical curiosities containing more dissolved solute than should theoretically be possible. They're like an overpacked car park that somehow fits extra cars. The slightest disturbance or addition of a tiny crystal causes excess solute to crash out of solution dramatically.
Understanding these classifications helps predict how solutions will behave during analytical procedures. In gravimetric analysis, you often work with saturated solutions to ensure complete precipitation.
Visual Memory: Picture the three beakers shown - unsaturated (plenty of room), saturated (perfectly full), supersaturated (dangerously overfull)!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
rafallaith
@rafallaith_9djz3m5vk
Analytical chemistry is the detective work of the scientific world - it's all about figuring out what's in a sample and how much of it is there. This branch of chemistry splits into two main approaches: qualitative analysis (identifying what's... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This is your introduction to analytical chemistry, taught by Dr. Jwan Oday Abdulsattar. You'll be exploring the fundamental concepts that help scientists identify and measure chemical compounds in everything from medical samples to crime scene evidence.
The course combines theoretical knowledge with practical mathematical problem-solving skills. You'll discover how analytical chemistry connects to real-world applications across medicine, forensics, and industry.
Quick Tip: Think of analytical chemistry as being like a detective - you're gathering clues to solve chemical mysteries!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Analytical chemistry is essentially the science of answering two crucial questions: "What's in this sample?" and "How much is there?" It involves three key processes: separation, identification, and quantification of chemical compounds.
The field splits into two main branches. Qualitative analysis tells you what's present in a sample - think of it as making a list of ingredients. Quantitative analysis measures exactly how much of each component is there - like getting precise measurements for a recipe.
This isn't just academic theory - analytical chemistry powers everything from diabetes testing to solving crimes. Doctors use it for medical diagnoses, forensic scientists analyse evidence, and engineers ensure product quality. Qualitative chemical analysis specifically focuses on identifying whether ions, atoms, or compounds are present or absent.
Before starting any analysis, you need to determine if your sample contains organic compounds or inorganic compounds . This classification determines which analytical methods you'll use.
Real-World Connection: Every time you take a home pregnancy test or check your blood sugar, you're using analytical chemistry principles!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
You don't always need expensive equipment for qualitative analysis - sometimes your senses are enough! Simple identification can be based on observable properties like colour, odour, melting point, boiling point, texture, and electrical conductivity.
The most common approach involves adding chemical reagents to your sample and watching what happens. These reagents are chosen to react selectively with specific compounds, usually producing either a coloured complex or a precipitate that you can easily spot.
Think about a urine test - when you add the test reagent, colour changes indicate the presence of substances like glucose. High glucose levels suggest diabetes, but you'd need a quantitative blood test to get exact concentration values. Another classic example is testing for copper: when copper(II) reacts with ammonia, it forms a distinctive deep blue copper-ammonia complex.
The limitation of classical qualitative chemical analysis is that it usually only identifies parts of molecules rather than complete structures. That's why analysts often combine these tests with measurements of boiling points, melting points, and densities to get the full picture.
Study Hack: Remember that qualitative = quality (what type), not quantity (how much)!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Qualitative chemical analysis shines brightest in medical and forensic applications. Doctors rely on it for diagnostic tests - from checking blood chemistry to identifying infections. It's often the first step before more detailed quantitative testing.
In criminology, forensic scientists use these techniques to identify mysterious substances found at crime scenes. A simple colour test might reveal whether a white powder is cocaine or baking soda, potentially making or breaking a criminal case.
You've probably used qualitative analysis at home without realising it. When you check if margarine melts faster than butter, or test whether a cleaning product changes colour on different surfaces, you're doing basic qualitative analysis.
The beauty of this field is its immediate, practical nature. Unlike some areas of chemistry that seem purely theoretical, qualitative analysis gives you instant, visible results that have real-world consequences.
Career Insight: Qualitative analysis skills are essential for careers in medicine, forensic science, environmental monitoring, and quality control!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
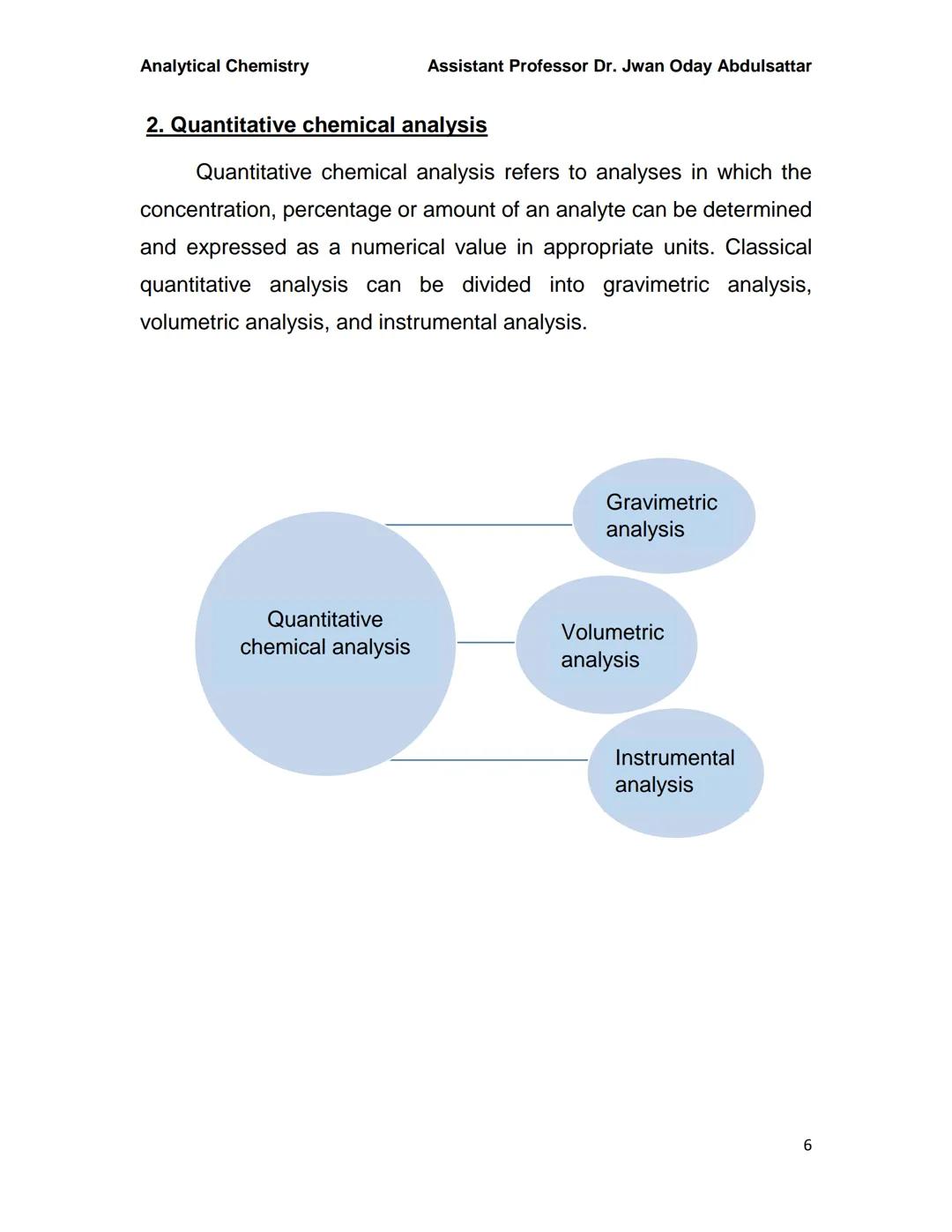
This page provides a visual overview of chemical analysis structure, showing how the field branches into qualitative and quantitative approaches.
Qualitative analysis forms the foundation - it's your starting point for understanding what you're dealing with before you can measure it accurately.
The diagram helps you visualise the systematic approach that analytical chemists use, moving from general identification to specific quantification based on what the situation requires.
Visual Learner Tip: Use this diagram as a mental map when tackling analytical chemistry problems!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Quantitative chemical analysis is where you get the numbers - determining exactly how much of a substance is present in your sample. Unlike qualitative analysis, this gives you measurable results expressed in units like grams, percentages, or concentrations.
The field divides into three main approaches: gravimetric analysis (measuring mass), volumetric analysis (measuring volume), and instrumental analysis (using specialised equipment).
Each method has its strengths depending on what you're measuring and how precise you need to be. Gravimetric analysis is incredibly accurate but time-consuming, while volumetric analysis is faster and still very reliable.
Understanding when to use each technique is crucial for any analytical chemist. Your choice depends on factors like the sample type, required accuracy, available time, and equipment.
Exam Tip: Learn the three types of quantitative analysis and when to use each one - this often appears in exam questions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
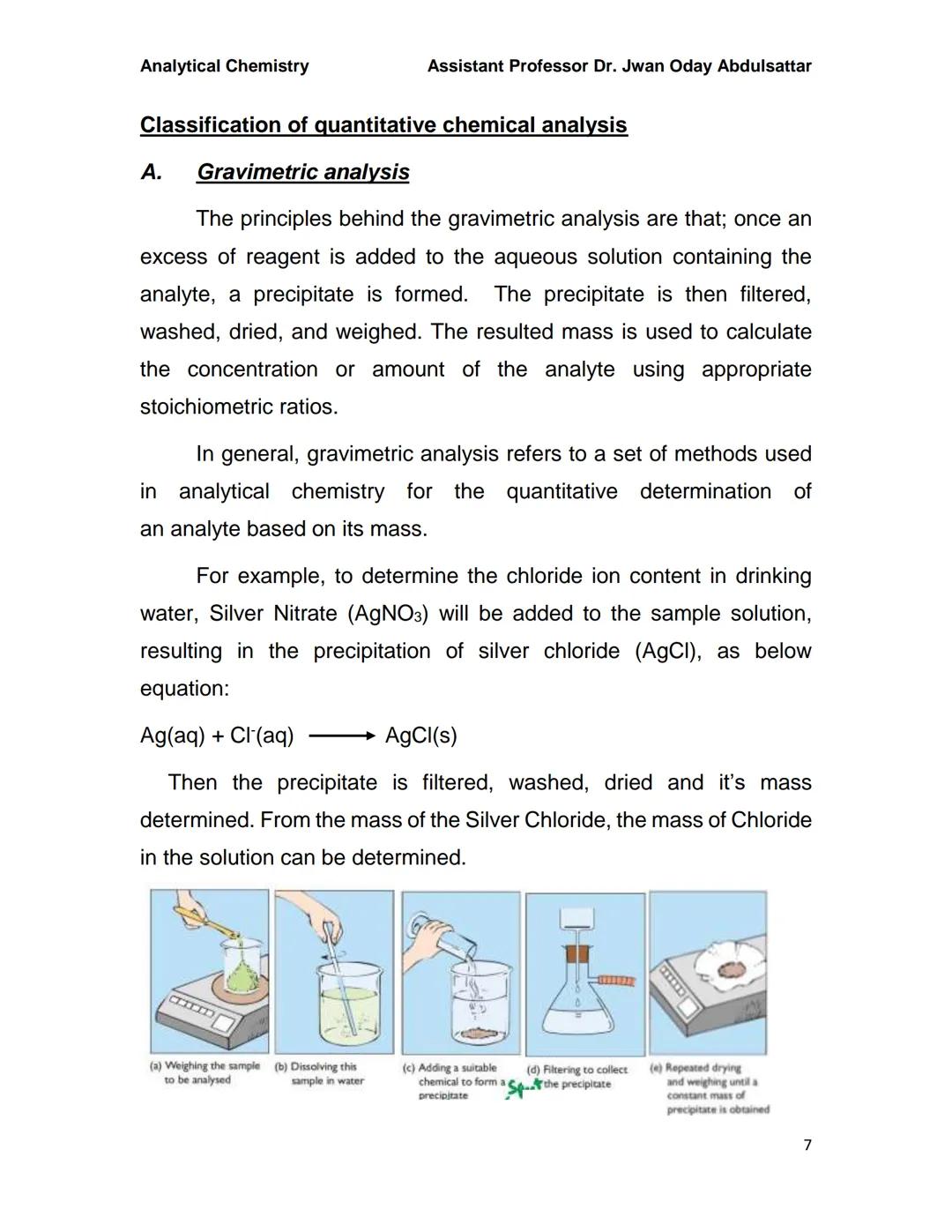
Gravimetric analysis is beautifully simple in principle: you add a reagent that makes your target substance form a precipitate, then you filter, wash, dry, and weigh it. The mass tells you exactly how much of your original substance was present.
Here's how it works: add excess reagent to your sample solution, causing precipitation. Filter out the solid, wash it thoroughly to remove impurities, dry it completely, then weigh it. Using stoichiometric ratios, you can calculate back to find your original concentration.
Take measuring chloride in drinking water as an example. Add silver nitrate (AgNO₃) and watch silver chloride (AgCl) precipitate out: Ag⁺(aq) + Cl⁻(aq) → AgCl(s). Weigh the AgCl precipitate, and you can calculate exactly how much chloride was in your water sample.
The process involves five clear steps: weighing your sample, dissolving it, adding precipitating reagent, filtering the precipitate, and repeated drying/weighing until you get a constant mass. This method is incredibly accurate but requires patience and careful technique.
Lab Success: Always dry your precipitate to constant mass - this means weighing, drying, and reweighing until the mass doesn't change!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Volumetric analysis (also called titrimetric analysis) is like a chemical balancing act. You gradually add a solution of known concentration (the titrant) to your unknown sample until you reach the equivalence point - where they're perfectly balanced chemically.
The key players are primary standard solutions and secondary standard solutions. Primary standards like sodium carbonate (Na₂CO₃) are ultra-pure reference materials that meet strict criteria: high purity, air stability, no water content, readily available, reasonably soluble, and large molar mass to minimise weighing errors.
Secondary standards are prepared in the lab and standardised against primary standards. Sodium hydroxide is a classic example - commercial NaOH contains impurities and absorbs water from air, so it can't be a primary standard.
The magic happens at the equivalence point, where moles of titrant equal moles of analyte. You'll often see this as a colour change, either natural or from an added indicator. The endpoint (where you see the colour change) approximates the equivalence point and lets you calculate your unknown concentration.
Practical Tip: The equivalence point is theoretical perfection; the endpoint is what you actually observe - they're close but not identical!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Biological titration shows how versatile volumetric analysis can be. In vaccine production, scientists use serial dilutions (1:1, 1:2, 1:4, 1:8) to count viruses and determine the titer - the dilution limit where viruses are still detectable.
Understanding solutions is fundamental to all this work. A solution is simply a homogeneous mixture where the solute (dissolved substance) disperses evenly throughout the solvent (the dissolving medium). Think salt (solute) dissolving in water (solvent) to make salt water.
The process is straightforward: NaCl(s) + H₂O(l) → Salt solution. The solid salt breaks apart and spreads uniformly throughout the water, creating a solution where every drop contains the same concentration.
These concepts underpin every quantitative analysis you'll perform. Whether you're measuring medicine concentrations or testing water purity, you're working with solutions and applying these same principles.
Memory Aid: Solute = dissolved (like sugar), Solvent = dissolves (like water), Solution = the final mixture!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Solutions behave differently depending on how much solute they contain. Unsaturated solutions have room for more solute - everything dissolves completely with no leftovers. It's like a car park with plenty of empty spaces.
Saturated solutions contain the maximum possible amount of dissolved solute at that temperature. Add more solute and it simply won't dissolve - it'll sit at the bottom like cars circling a full car park. This represents the natural solubility limit.
Supersaturated solutions are unstable chemical curiosities containing more dissolved solute than should theoretically be possible. They're like an overpacked car park that somehow fits extra cars. The slightest disturbance or addition of a tiny crystal causes excess solute to crash out of solution dramatically.
Understanding these classifications helps predict how solutions will behave during analytical procedures. In gravimetric analysis, you often work with saturated solutions to ensure complete precipitation.
Visual Memory: Picture the three beakers shown - unsaturated (plenty of room), saturated (perfectly full), supersaturated (dangerously overfull)!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
11
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user