Ready to dive into some seriously cool chemistry? These pages... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
210
•
13 Feb 2026
•
megan
@meganreid_imme
Ready to dive into some seriously cool chemistry? These pages... Show more




Ever wondered how scientists figure out what stars are made of? It's all about light and energy levels in atoms! When you heat up elements, their electrons get excited and jump to higher energy levels - like students moving up to harder classes.
When these excited electrons fall back down, they release photons of specific colours. Each element has its own unique fingerprint of colours, which is brilliant for identification. Hydrogen produces different colours than sodium, for example.
There are two main techniques you need to know: Atomic Emission Spectroscopy (AES) measures the light given off when electrons fall down energy levels, while Atomic Absorption Spectroscopy (AAS) measures which wavelengths get absorbed. The intensity tells you how much of an element is present - dead useful for analysis!
The key equation connecting energy and light is E = hf, where h is Planck's constant (6.63×10⁻³⁴ J·s). Light behaves as both waves and particles (called photons), which might sound mental but it's absolutely fundamental to understanding atomic behaviour.
💡 Quick Tip: Remember that the further electrons are from the nucleus, the less energy difference between levels - this affects which colours you'll see!
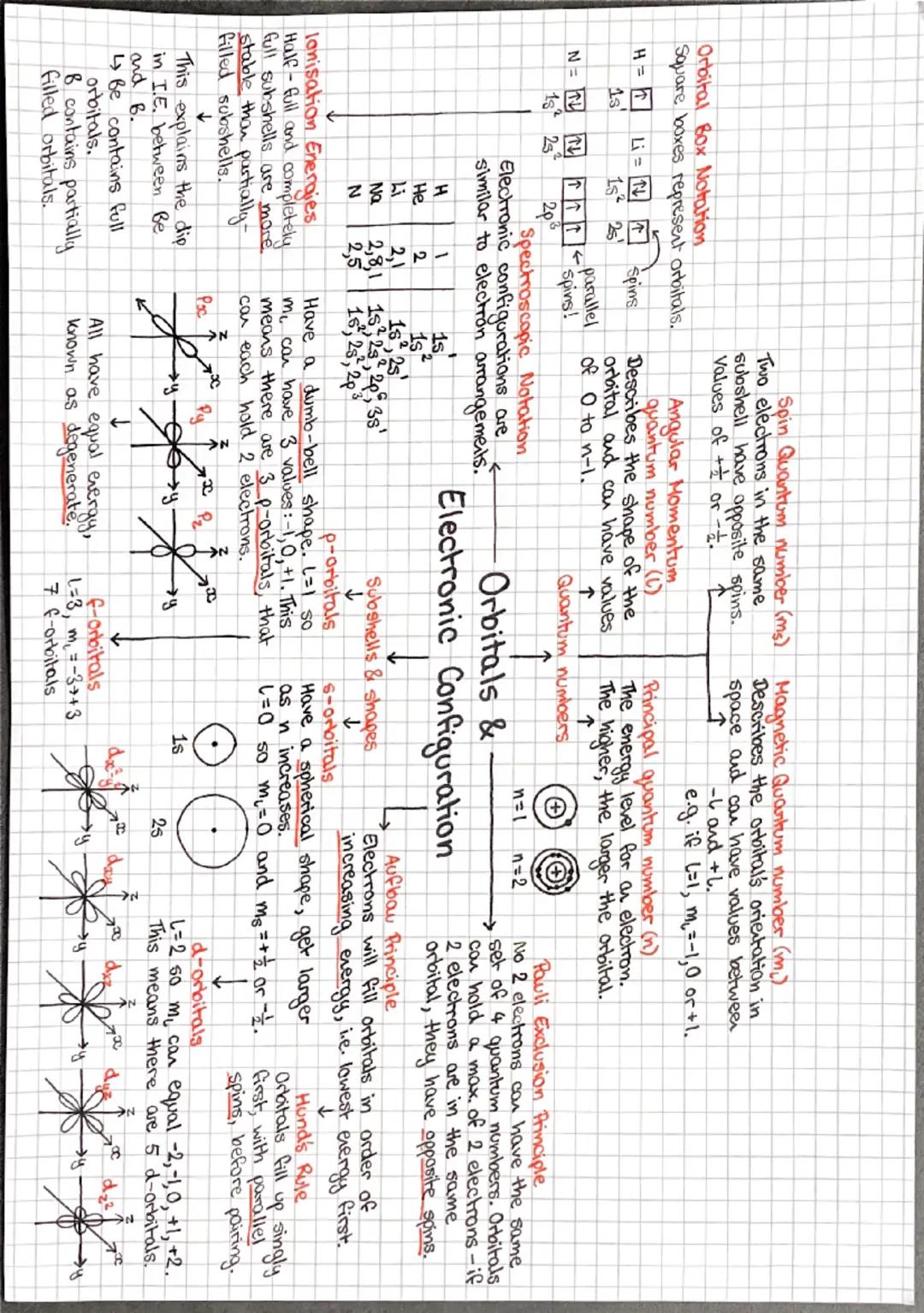
Your electron arrangements from earlier years were just the beginning - now we're getting into the proper detail with quantum numbers and orbital shapes! Think of orbitals as the specific "rooms" where electrons live around an atom.
S-orbitals are spherical (like footballs), p-orbitals are dumbbell-shaped, and d-orbitals have more complex shapes. Each type can hold different numbers of electrons: s holds 2, p holds 6, and d holds 10. The principal quantum number (n) tells you the energy level.
Three crucial rules govern how electrons fill orbitals: Aufbau principle (lowest energy first), Pauli exclusion principle (maximum 2 electrons per orbital with opposite spins), and Hund's rule (fill singly before pairing up). These explain why ionisation energies don't always increase smoothly across periods.
Half-filled and completely filled subshells are particularly stable, which explains some unexpected electron configurations. For instance, chromium prefers [Ar] 3d⁵ 4s¹ rather than [Ar] 3d⁴ 4s² because the half-filled d-shell provides extra stability.
💡 Quick Tip: Use orbital box notation to visualise electron arrangements - it makes predicting magnetic properties and reactivity much clearer!
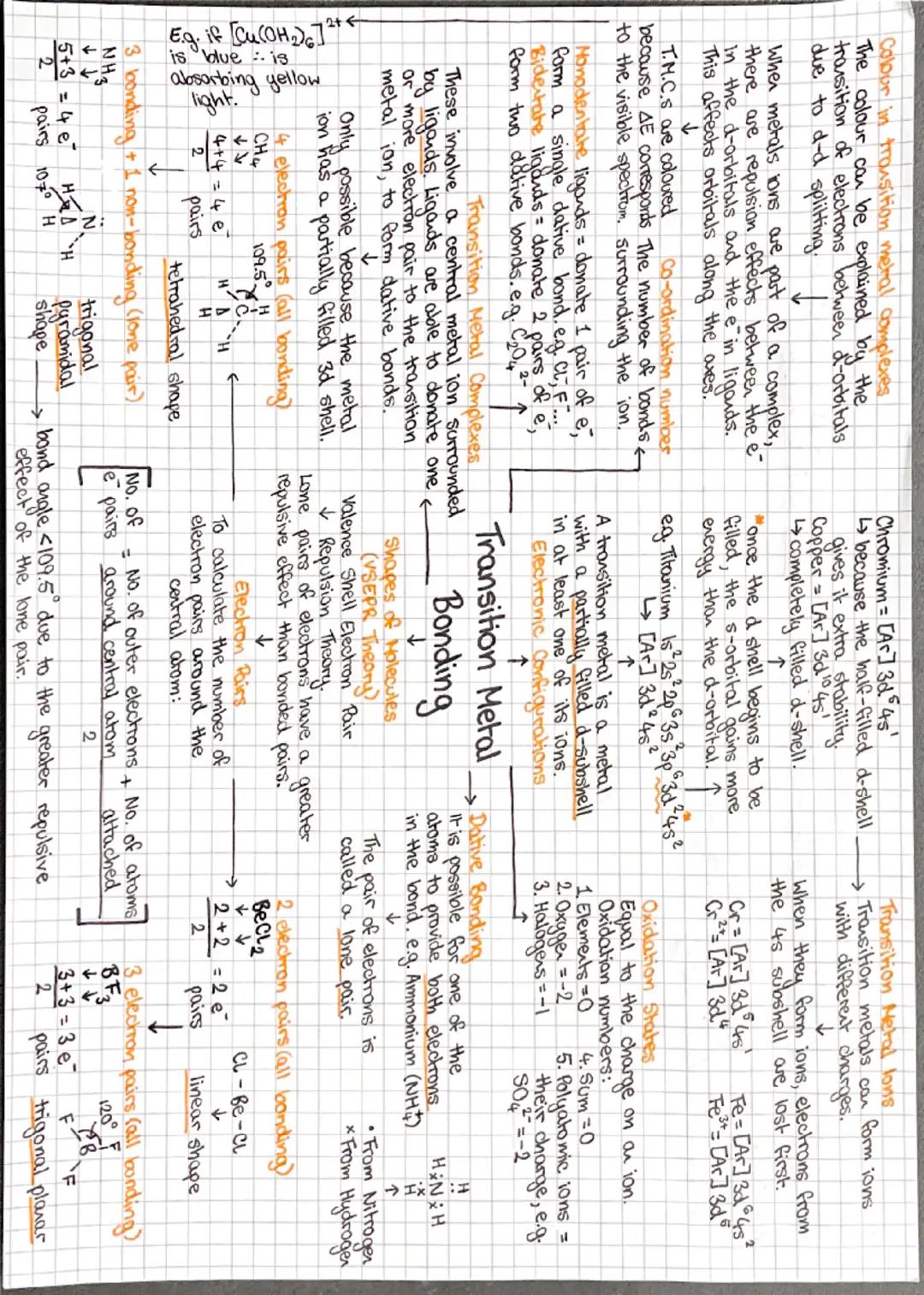
VSEPR theory is your best mate for predicting molecular shapes - electrons hate each other and spread out as far as possible! Count up electron pairs around the central atom: 2 pairs = linear, 3 pairs = trigonal planar, 4 pairs = tetrahedral.
Lone pairs are bullies - they repel more strongly than bonding pairs, so they squash bond angles. That's why ammonia (with one lone pair) has a bond angle less than 109.5°, making it trigonal pyramidal rather than tetrahedral.
Transition metals are the colourful characters of chemistry! They form complex ions where ligands (electron pair donors) surround a central metal ion through dative bonding. The gorgeous colours come from d-d electron transitions when light hits these complexes.
These metals can form multiple oxidation states because they lose 4s electrons first, then d electrons. When forming complexes, the d-orbitals split due to ligand repulsion, creating an energy gap that corresponds perfectly to visible light wavelengths.
💡 Quick Tip: Only transition metals with partially filled d-shells show colour - if the d-shell is empty or completely full, no d-d transitions can occur!

Dynamic equilibrium isn't about nothing happening - it's like a busy roundabout where cars enter and leave at exactly the same rate! The forward and reverse reaction rates become equal, but molecules are still reacting constantly.
The equilibrium constant (K) tells you who's winning the battle between reactants and products. If K > 1, products dominate; if K < 1, reactants are in charge. Temperature is the only thing that actually changes K values.
Le Chatelier's principle is your prediction tool: disturb an equilibrium and it shifts to minimise that disturbance. Add more reactant? The equilibrium shifts right to use it up. Increase pressure? It shifts towards fewer gas molecules.
ICE calculations (Initial, Change, Equilibrium) help you work out concentrations and K values. Set up a table, use algebra to track the changes, and substitute into the K expression. For temperature effects, remember: increasing temperature favours the endothermic direction.
💡 Quick Tip: Only include gases and aqueous species in K expressions - pure solids and liquids have constant concentrations so they're ignored!
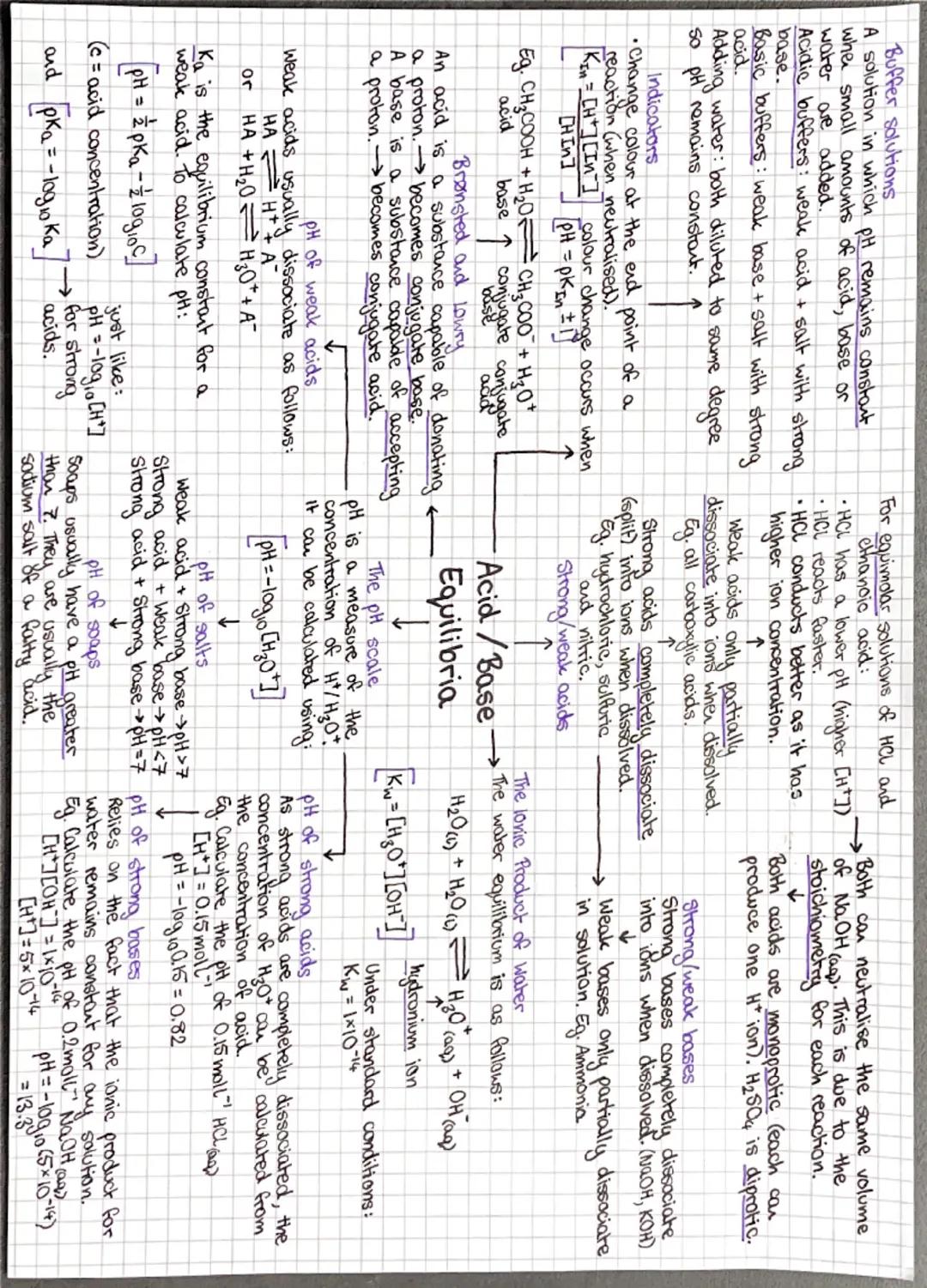
Forget the old "acids taste sour" definition - Brønsted-Lowry theory is where it's at! Acids are proton (H⁺) donors, bases are proton acceptors. When they react, they form conjugate acid-base pairs.
Strong acids like HCl completely dissociate in water, so pH calculations are straightforward: pH = -log[H⁺]. Weak acids only partially dissociate, so you need the acid dissociation constant Ka and the equation pH = -log Ka - log.
The ionic product of water is absolutely crucial. It connects [H⁺] and [OH⁻] in any aqueous solution, letting you calculate pH of bases too. For strong bases, work out [OH⁻], then use Kw to find [H⁺].
Buffer solutions resist pH changes by containing a weak acid and its conjugate base. They work by neutralising small amounts of added acid or base. The Henderson-Hasselbalch equation helps calculate buffer pH.
💡 Quick Tip: Indicators change colour at their pKa value - choose one whose pKa matches your expected equivalence point pH for sharp colour changes!
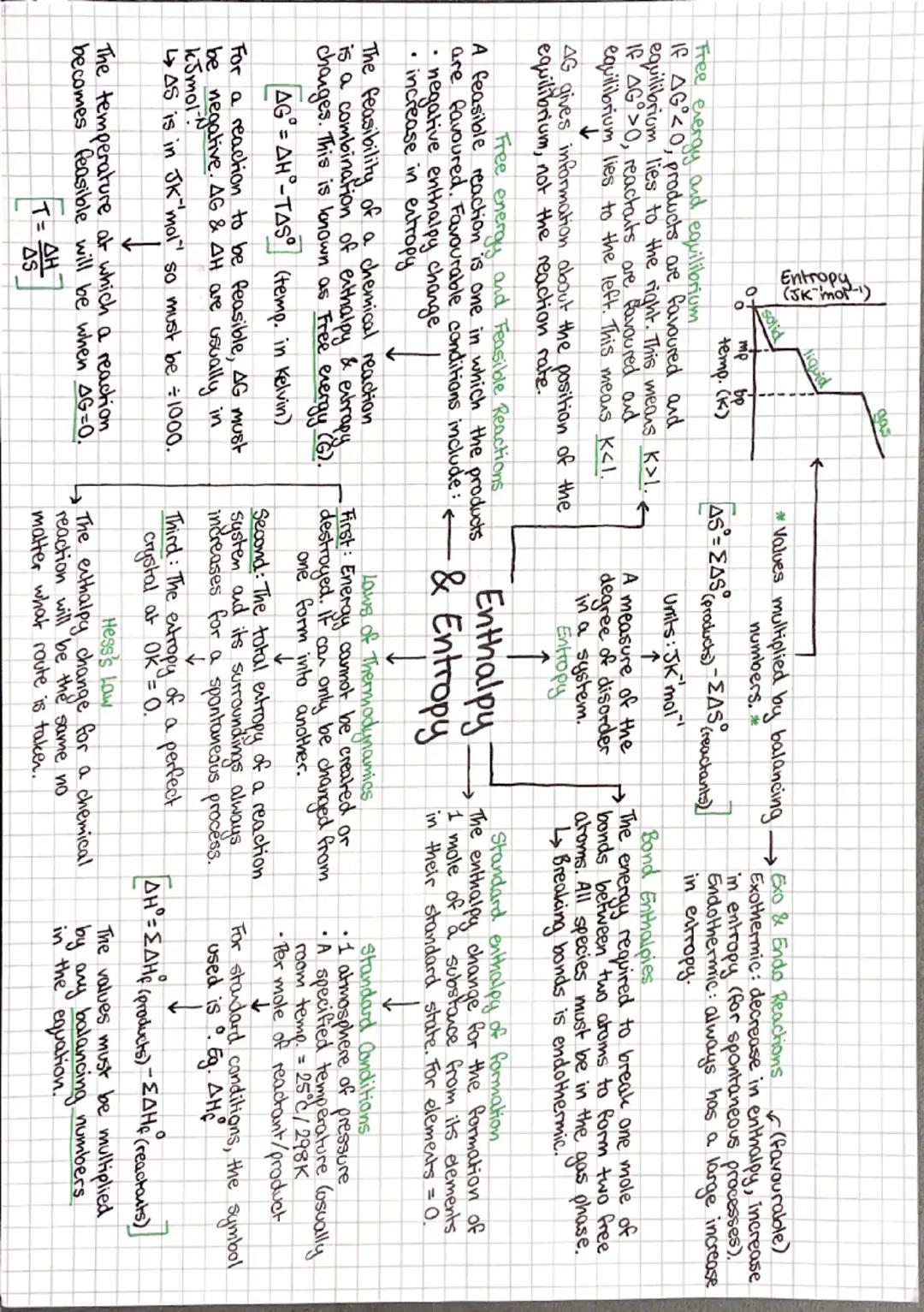
Gibbs free energy (ΔG) is the ultimate judge of whether reactions can happen spontaneously. The equation ΔG = ΔH - TΔS combines enthalpy and entropy effects. If ΔG is negative, the reaction is feasible - products are favoured.
Entropy measures disorder, and the universe loves chaos! Solids have low entropy, gases have high entropy. When calculating ΔS, remember that reactions producing more gas molecules usually increase entropy significantly.
Temperature can make or break reaction feasibility. For endothermic reactions with positive ΔS, there's a threshold temperature where ΔG becomes negative. Set ΔG = 0 and solve: T = ΔH/ΔS gives you this crossover point.
Standard enthalpy of formation values let you calculate ΔH using Hess's law: ΔH = Σ ΔHf(products) - Σ ΔHf(reactants). Elements in their standard states have ΔHf = 0, which simplifies calculations loads.
💡 Quick Tip: Watch your units! ΔH is usually in kJ/mol but ΔS is in J/K·mol - convert ΔS by dividing by 1000 to match units in the Gibbs equation.

Collision theory explains why reactions have different speeds - particles need enough kinetic energy and the right orientation to react successfully. It's like trying to unlock your phone while running for the bus!
The rate-determining step (RDS) is the slowest step in any multi-step reaction mechanism. Think of it as the bottleneck that controls the overall reaction rate. The rate equation depends only on species involved in this slow step.
Reaction orders show how concentration changes affect rate. Zero order means concentration has no effect, first order means doubling concentration doubles the rate, and second order means doubling concentration quadruples the rate.
The rate constant (k) depends on temperature and tells you how fast a reaction proceeds at given concentrations. Its units change with overall reaction order: mol⁻¹s⁻¹ for second order, s⁻¹ for first order, mol·s⁻¹ for zero order.
Rate-concentration graphs have the steepest gradient at t = 0 (the initial rate) because this is when reactant concentrations are highest. As reactions progress, rates slow down because there's less reactant left to collide.
💡 Quick Tip: You can't predict reaction orders from balanced equations - they must be determined experimentally by measuring how rate changes with concentration!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
megan
@meganreid_imme
Ready to dive into some seriously cool chemistry? These pages cover everything from how light reveals the secrets of atoms to why reactions happen at different speeds - basically all the chemistry knowledge that'll make you sound like a proper... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered how scientists figure out what stars are made of? It's all about light and energy levels in atoms! When you heat up elements, their electrons get excited and jump to higher energy levels - like students moving up to harder classes.
When these excited electrons fall back down, they release photons of specific colours. Each element has its own unique fingerprint of colours, which is brilliant for identification. Hydrogen produces different colours than sodium, for example.
There are two main techniques you need to know: Atomic Emission Spectroscopy (AES) measures the light given off when electrons fall down energy levels, while Atomic Absorption Spectroscopy (AAS) measures which wavelengths get absorbed. The intensity tells you how much of an element is present - dead useful for analysis!
The key equation connecting energy and light is E = hf, where h is Planck's constant (6.63×10⁻³⁴ J·s). Light behaves as both waves and particles (called photons), which might sound mental but it's absolutely fundamental to understanding atomic behaviour.
💡 Quick Tip: Remember that the further electrons are from the nucleus, the less energy difference between levels - this affects which colours you'll see!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
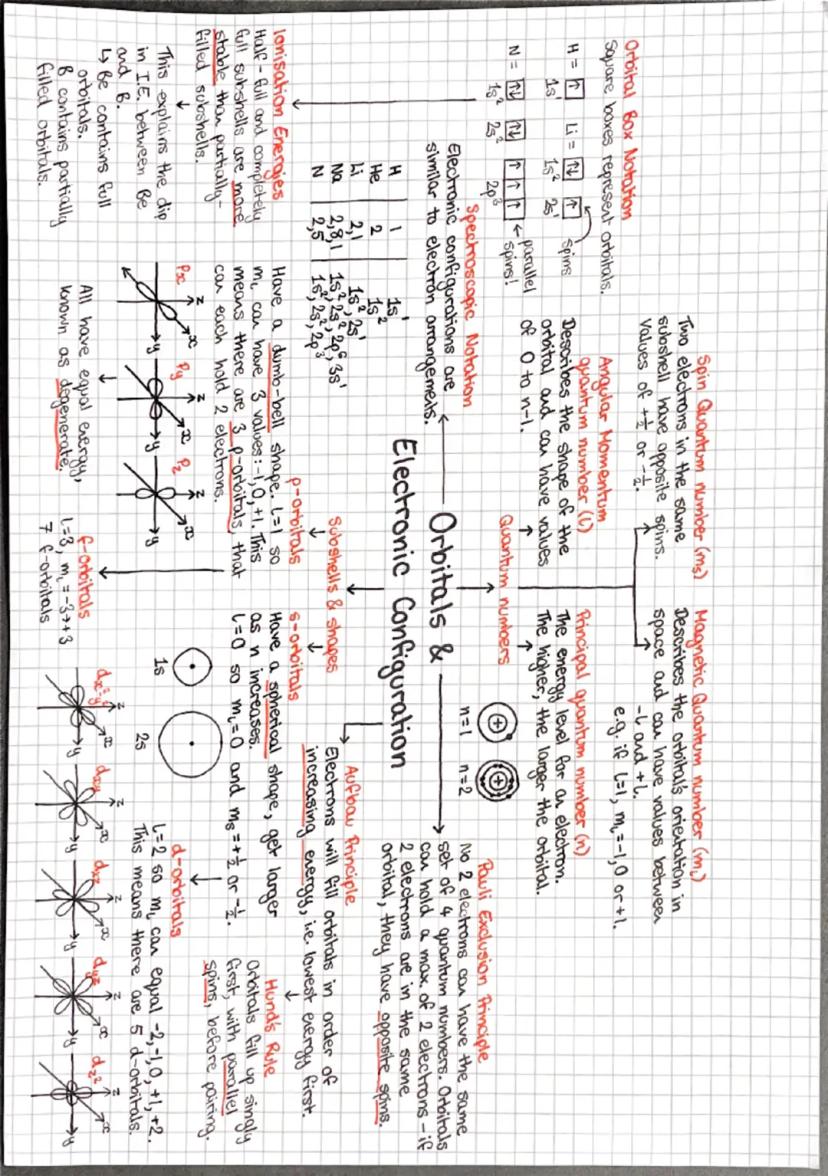
Your electron arrangements from earlier years were just the beginning - now we're getting into the proper detail with quantum numbers and orbital shapes! Think of orbitals as the specific "rooms" where electrons live around an atom.
S-orbitals are spherical (like footballs), p-orbitals are dumbbell-shaped, and d-orbitals have more complex shapes. Each type can hold different numbers of electrons: s holds 2, p holds 6, and d holds 10. The principal quantum number (n) tells you the energy level.
Three crucial rules govern how electrons fill orbitals: Aufbau principle (lowest energy first), Pauli exclusion principle (maximum 2 electrons per orbital with opposite spins), and Hund's rule (fill singly before pairing up). These explain why ionisation energies don't always increase smoothly across periods.
Half-filled and completely filled subshells are particularly stable, which explains some unexpected electron configurations. For instance, chromium prefers [Ar] 3d⁵ 4s¹ rather than [Ar] 3d⁴ 4s² because the half-filled d-shell provides extra stability.
💡 Quick Tip: Use orbital box notation to visualise electron arrangements - it makes predicting magnetic properties and reactivity much clearer!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
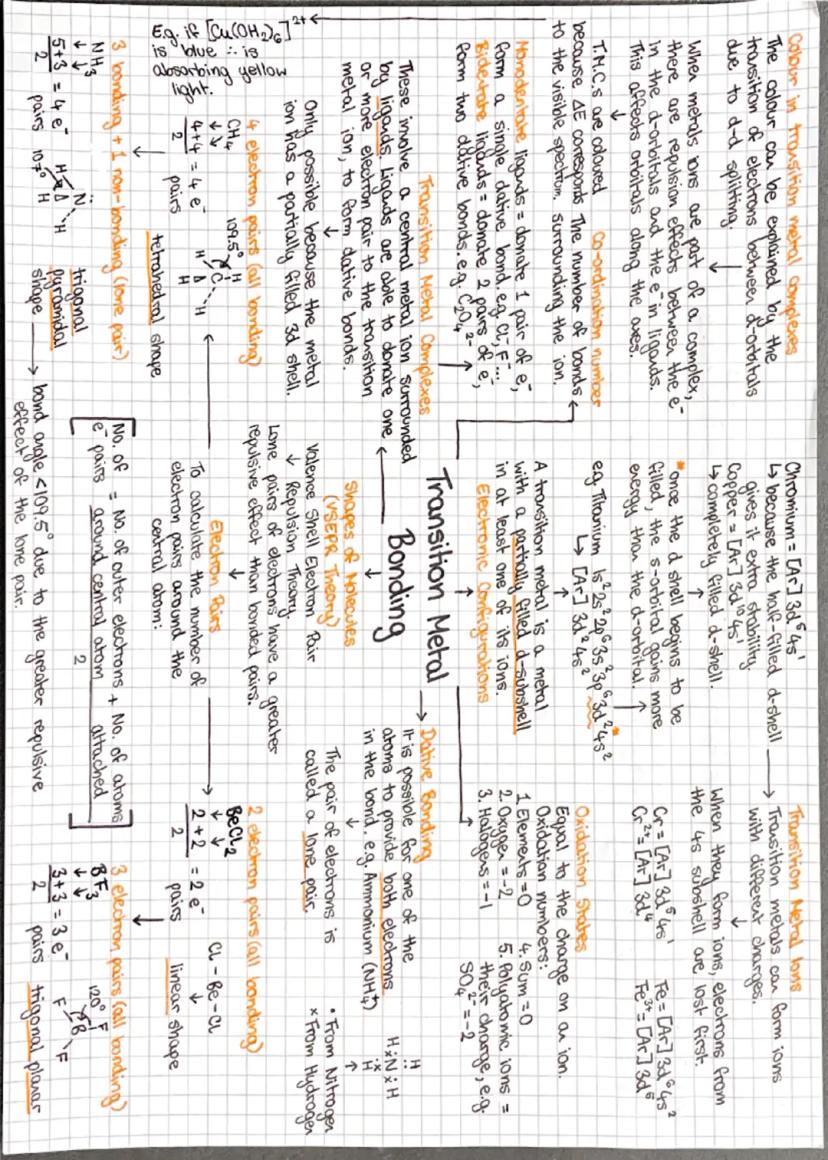
VSEPR theory is your best mate for predicting molecular shapes - electrons hate each other and spread out as far as possible! Count up electron pairs around the central atom: 2 pairs = linear, 3 pairs = trigonal planar, 4 pairs = tetrahedral.
Lone pairs are bullies - they repel more strongly than bonding pairs, so they squash bond angles. That's why ammonia (with one lone pair) has a bond angle less than 109.5°, making it trigonal pyramidal rather than tetrahedral.
Transition metals are the colourful characters of chemistry! They form complex ions where ligands (electron pair donors) surround a central metal ion through dative bonding. The gorgeous colours come from d-d electron transitions when light hits these complexes.
These metals can form multiple oxidation states because they lose 4s electrons first, then d electrons. When forming complexes, the d-orbitals split due to ligand repulsion, creating an energy gap that corresponds perfectly to visible light wavelengths.
💡 Quick Tip: Only transition metals with partially filled d-shells show colour - if the d-shell is empty or completely full, no d-d transitions can occur!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Dynamic equilibrium isn't about nothing happening - it's like a busy roundabout where cars enter and leave at exactly the same rate! The forward and reverse reaction rates become equal, but molecules are still reacting constantly.
The equilibrium constant (K) tells you who's winning the battle between reactants and products. If K > 1, products dominate; if K < 1, reactants are in charge. Temperature is the only thing that actually changes K values.
Le Chatelier's principle is your prediction tool: disturb an equilibrium and it shifts to minimise that disturbance. Add more reactant? The equilibrium shifts right to use it up. Increase pressure? It shifts towards fewer gas molecules.
ICE calculations (Initial, Change, Equilibrium) help you work out concentrations and K values. Set up a table, use algebra to track the changes, and substitute into the K expression. For temperature effects, remember: increasing temperature favours the endothermic direction.
💡 Quick Tip: Only include gases and aqueous species in K expressions - pure solids and liquids have constant concentrations so they're ignored!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
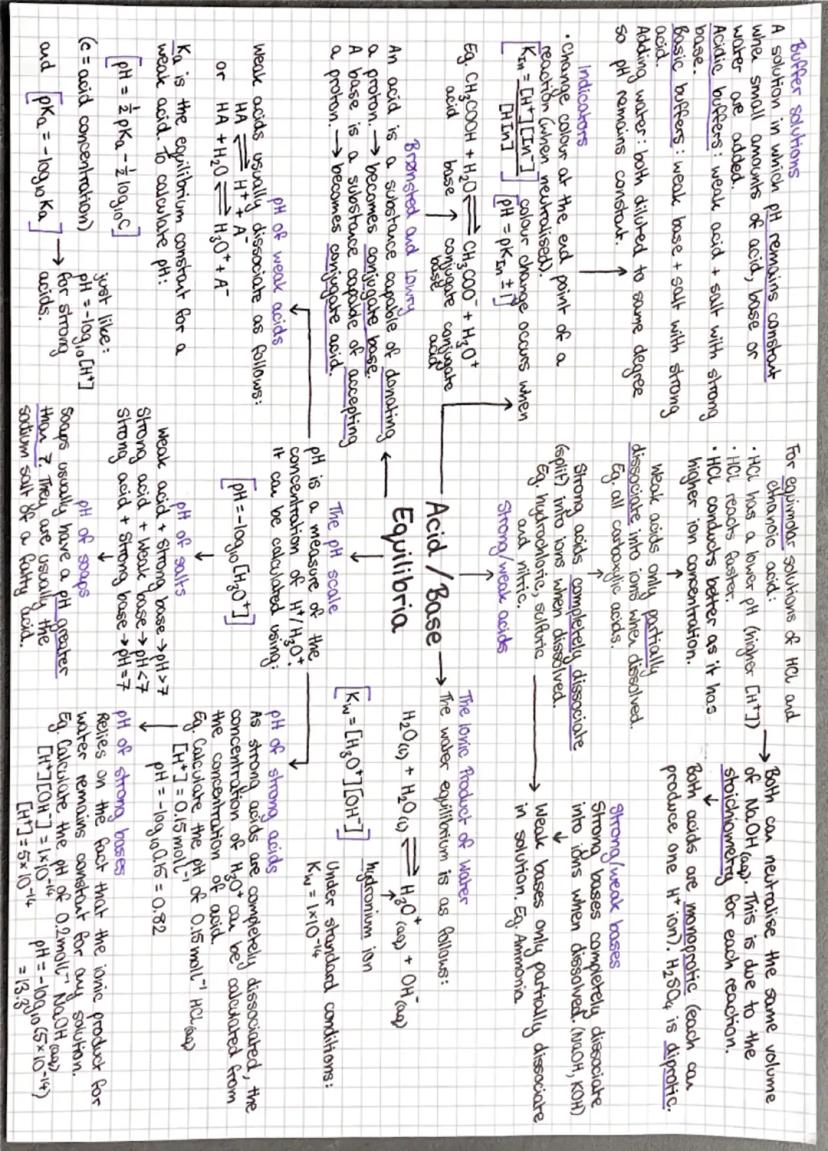
Forget the old "acids taste sour" definition - Brønsted-Lowry theory is where it's at! Acids are proton (H⁺) donors, bases are proton acceptors. When they react, they form conjugate acid-base pairs.
Strong acids like HCl completely dissociate in water, so pH calculations are straightforward: pH = -log[H⁺]. Weak acids only partially dissociate, so you need the acid dissociation constant Ka and the equation pH = -log Ka - log.
The ionic product of water is absolutely crucial. It connects [H⁺] and [OH⁻] in any aqueous solution, letting you calculate pH of bases too. For strong bases, work out [OH⁻], then use Kw to find [H⁺].
Buffer solutions resist pH changes by containing a weak acid and its conjugate base. They work by neutralising small amounts of added acid or base. The Henderson-Hasselbalch equation helps calculate buffer pH.
💡 Quick Tip: Indicators change colour at their pKa value - choose one whose pKa matches your expected equivalence point pH for sharp colour changes!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
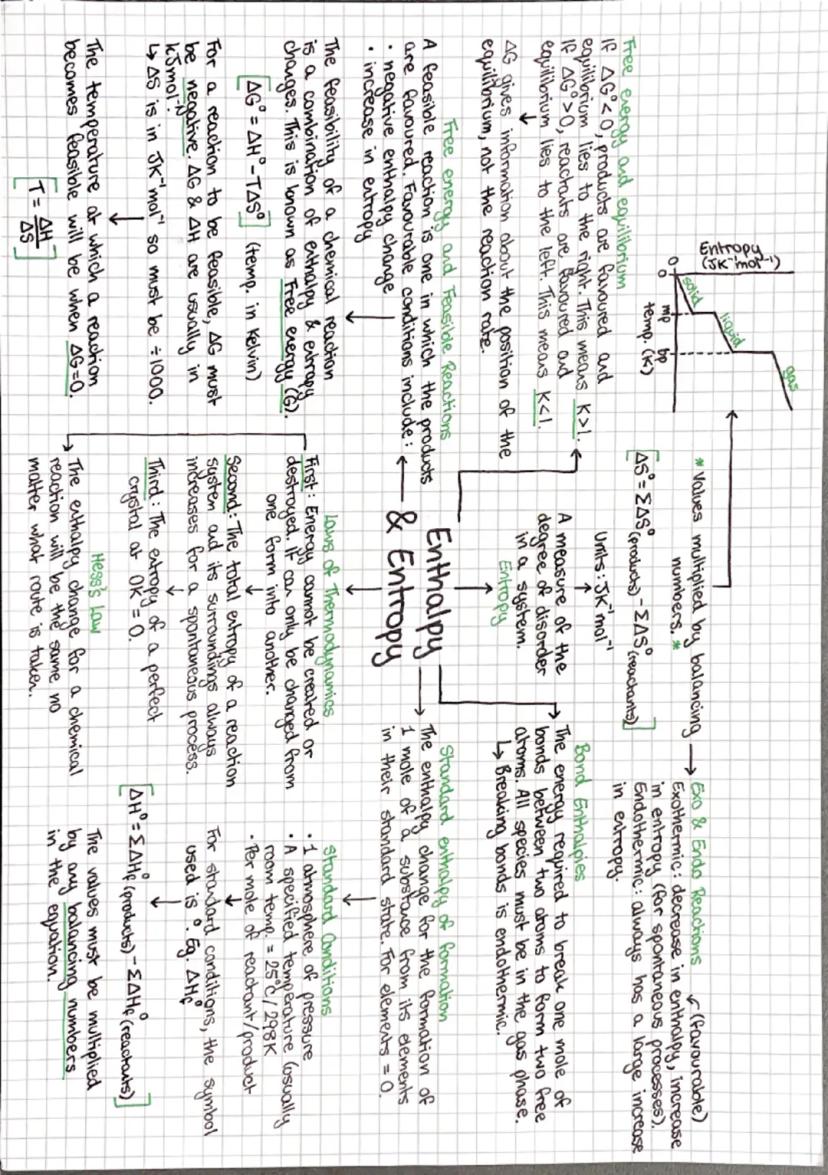
Gibbs free energy (ΔG) is the ultimate judge of whether reactions can happen spontaneously. The equation ΔG = ΔH - TΔS combines enthalpy and entropy effects. If ΔG is negative, the reaction is feasible - products are favoured.
Entropy measures disorder, and the universe loves chaos! Solids have low entropy, gases have high entropy. When calculating ΔS, remember that reactions producing more gas molecules usually increase entropy significantly.
Temperature can make or break reaction feasibility. For endothermic reactions with positive ΔS, there's a threshold temperature where ΔG becomes negative. Set ΔG = 0 and solve: T = ΔH/ΔS gives you this crossover point.
Standard enthalpy of formation values let you calculate ΔH using Hess's law: ΔH = Σ ΔHf(products) - Σ ΔHf(reactants). Elements in their standard states have ΔHf = 0, which simplifies calculations loads.
💡 Quick Tip: Watch your units! ΔH is usually in kJ/mol but ΔS is in J/K·mol - convert ΔS by dividing by 1000 to match units in the Gibbs equation.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Collision theory explains why reactions have different speeds - particles need enough kinetic energy and the right orientation to react successfully. It's like trying to unlock your phone while running for the bus!
The rate-determining step (RDS) is the slowest step in any multi-step reaction mechanism. Think of it as the bottleneck that controls the overall reaction rate. The rate equation depends only on species involved in this slow step.
Reaction orders show how concentration changes affect rate. Zero order means concentration has no effect, first order means doubling concentration doubles the rate, and second order means doubling concentration quadruples the rate.
The rate constant (k) depends on temperature and tells you how fast a reaction proceeds at given concentrations. Its units change with overall reaction order: mol⁻¹s⁻¹ for second order, s⁻¹ for first order, mol·s⁻¹ for zero order.
Rate-concentration graphs have the steepest gradient at t = 0 (the initial rate) because this is when reactant concentrations are highest. As reactions progress, rates slow down because there's less reactant left to collide.
💡 Quick Tip: You can't predict reaction orders from balanced equations - they must be determined experimentally by measuring how rate changes with concentration!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
9
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore Le Chatelier's Principle and its implications on chemical equilibrium in reversible reactions. This summary covers key concepts such as dynamic equilibrium, concentration shifts, and the behavior of systems in closed environments. Ideal for students seeking to understand how changes affect equilibrium states in chemistry.
Explore advanced concepts in chemical equilibrium, including equilibrium constants (Kc and Kp), the effects of concentration, pressure, and temperature changes on equilibrium, and the application of ICE tables. This summary is essential for A Level Chemistry students studying Edexcel Topic 11.
Explore the fundamentals of atomic orbitals, including their shapes, types (s, p, d, f), and the principles governing electron configurations. This summary covers key concepts such as quantum numbers, Hund's rule, the Aufbau principle, and the Pauli exclusion principle, essential for mastering OCR A Level Chemistry.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user