A-level Chemistry can feel overwhelming, but this cheat sheet pack... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
841
•
8 Feb 2026
•
k
@studywkay
A-level Chemistry can feel overwhelming, but this cheat sheet pack... Show more








This isn't just another revision guide - it's your complete toolkit for mastering A-level Chemistry. The pack condenses two years of study into focused, exam-ready summaries that highlight what actually matters for your grades.
Every cheat sheet maps directly to OCR specification points, so you won't waste time on irrelevant details. From basic atomic structure through to complex organic mechanisms, you've got everything covered in one place.
Quick Tip: Use this pack alongside your textbook, not as a replacement. It's designed to reinforce what you've learned and fill any gaps before exams.
The content spans all six modules of the OCR course, taking you from foundational chemistry concepts right through to advanced analytical techniques. Each section builds logically on the previous one, making revision feel less chaotic and more manageable.

This pack covers every major topic you'll encounter in A-level Chemistry, from atomic structure to chromatography. The table shows exactly how each cheat sheet aligns with OCR specification points, so you know you're studying the right content.
You'll notice topics are organised logically - starting with fundamental concepts like moles and equations before building up to complex areas like transition elements and organic synthesis. This structure mirrors how chemistry concepts actually connect in real life.
Study Smart: Focus on the specification points that appear most frequently in past papers - these tend to be the bread-and-butter topics that examiners love.
The pack includes everything from Group 2 reactions to NMR spectroscopy, with each section designed to be standalone yet interconnected. Whether you're cramming for mocks or doing final revision, you can jump to any topic and find what you need quickly.

Working with ionic compounds becomes straightforward once you understand the charge balancing game. Cations lose electrons , while anions gain them, and the overall compound must be electrically neutral.
Empirical formulas give you the simplest ratio of atoms, while molecular formulas tell the full story. To find empirical formulas, convert masses to moles, then divide by the smallest number - it's like finding the lowest common denominator in maths.
The mole concept is your gateway to quantitative chemistry. Remember that one mole always contains Avogadro's number of particles (6.022 × 10²³), whether you're counting atoms, molecules, or ions. Use n = m/M religiously.
Exam Focus: Percentage yield and atom economy questions are exam favourites. Higher atom economy means less waste and better environmental impact - perfect for those evaluation questions.
Gas calculations follow predictable patterns. Under standard conditions, one mole occupies 24 dm³, and the ideal gas equation PV = nRT handles everything else. Always check your units match the gas constant value you're using.

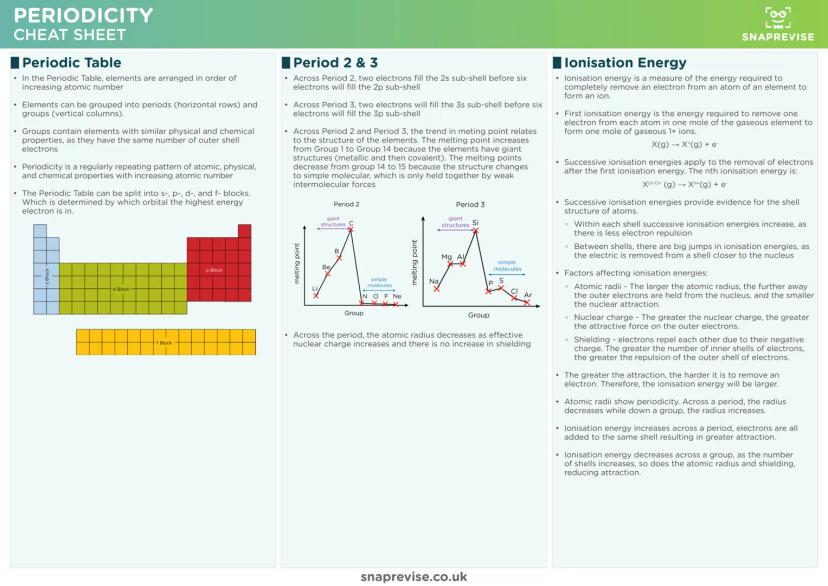
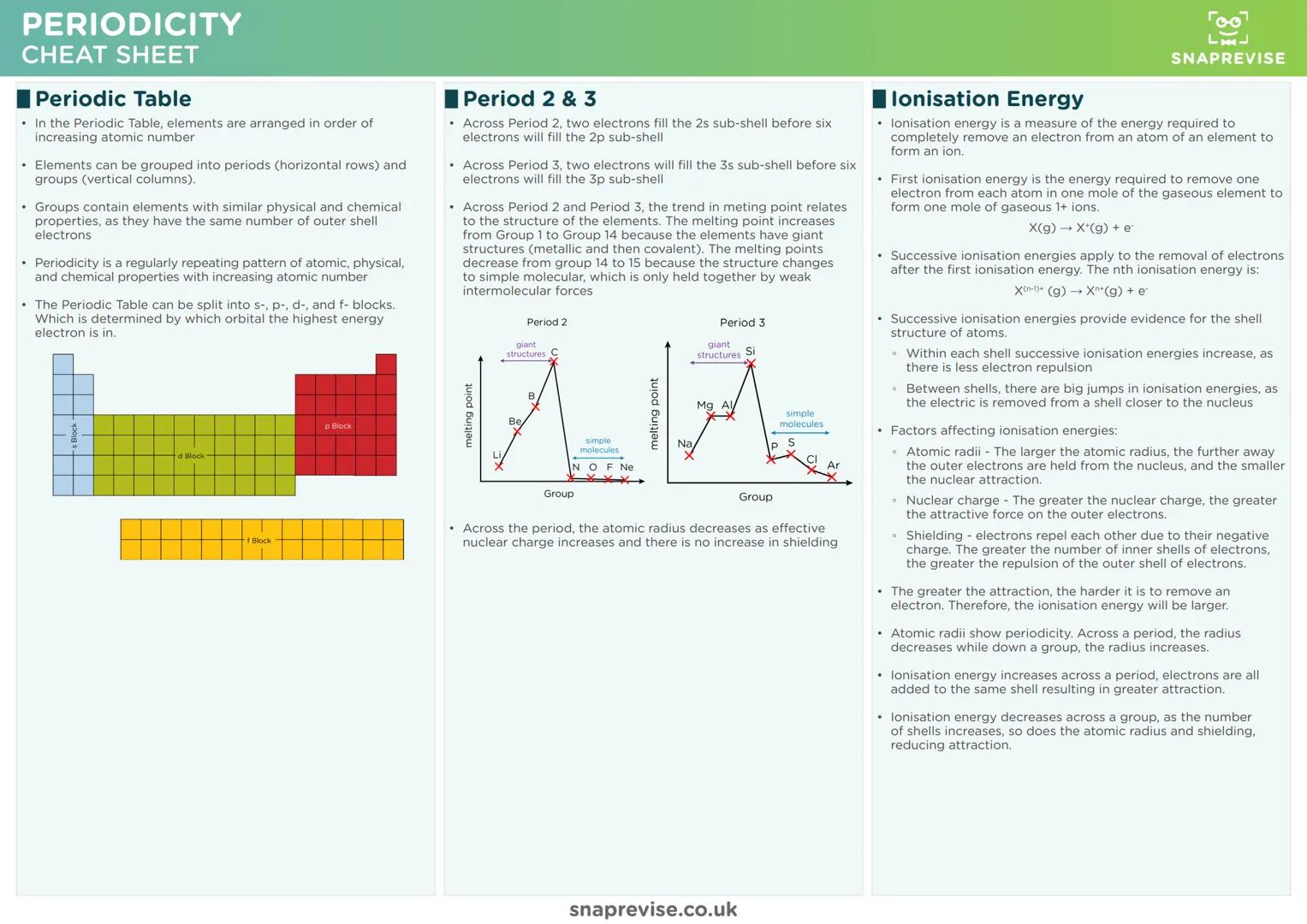
The Periodic Table isn't random - it's organised by increasing atomic number, creating predictable patterns called periodicity. Elements in the same group share similar properties because they have identical outer shell electron configurations.
Ionisation energy trends make perfect sense when you consider three key factors: nuclear charge, atomic radius, and electron shielding. Across periods, ionisation energy increases as nuclear charge grows stronger. Down groups, it decreases as electrons get further from the nucleus.
Successive ionisation energies provide brilliant evidence for electron shell structure. You'll see gradual increases within shells, then massive jumps when electrons are removed from inner shells closer to the nucleus.
Memory Aid: Think of ionisation energy like trying to pull someone away from a crowd - the closer they are to the centre (nucleus), the harder it becomes.
Atomic radius shows clear periodicity too. Across periods, radius decreases as increasing nuclear charge pulls electrons closer. Down groups, radius increases as extra electron shells are added, despite the stronger nucleus.

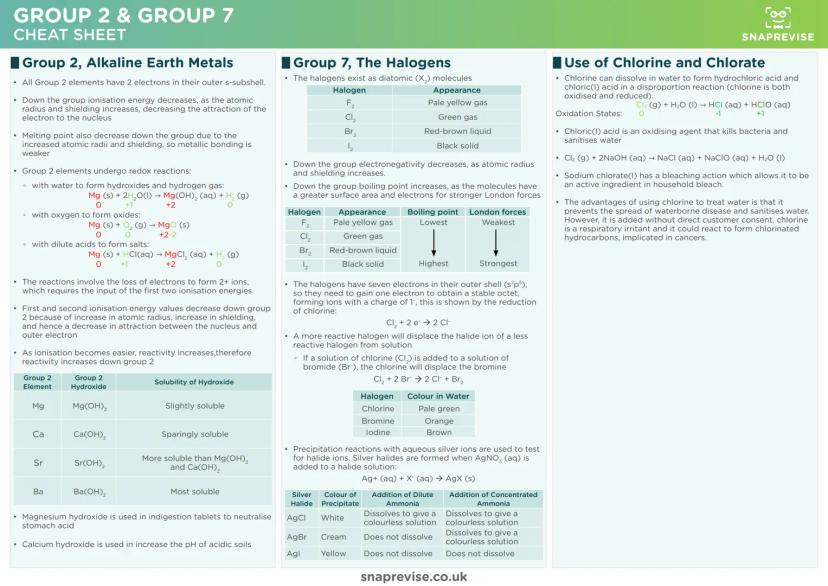
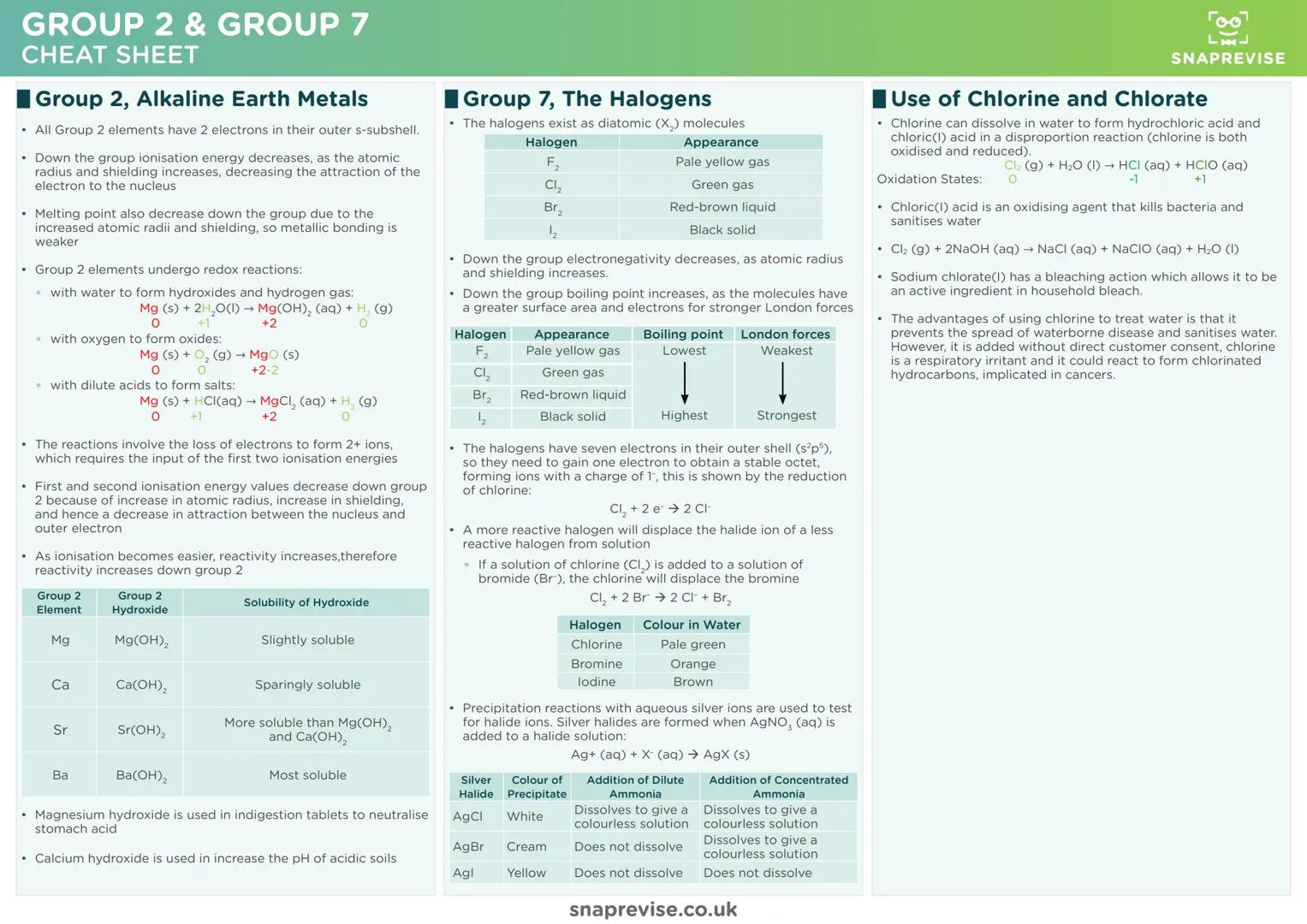
Group 2 elements (alkaline earth metals) become increasingly reactive down the group because ionisation energies decrease. As atoms get larger with more shielding, it's easier to remove those outer electrons and form 2+ ions.
These metals undergo predictable redox reactions - they lose electrons and get oxidised. Whether reacting with water, oxygen, or acids, Group 2 elements always form compounds where they have a +2 oxidation state.
Group 7 elements (halogens) exist as diatomic molecules and become less reactive down the group. As atomic radius increases, it becomes harder for these atoms to attract and gain the electron needed to complete their outer shell.
Test Tip: Learn the halide precipitation tests inside out. Silver chloride is white, silver bromide is cream, silver iodide is yellow - and their different solubilities in ammonia are exam gold.
Displacement reactions prove halogen reactivity order. More reactive halogens displace less reactive halide ions from solution - chlorine kicks out bromide and iodide, bromine displaces iodide, but iodine can't displace anything.
The chlorine-water equilibrium produces both HCl and HClO through disproportionation. This reaction is crucial for water treatment, though it comes with environmental and health considerations worth memorising for evaluation questions.

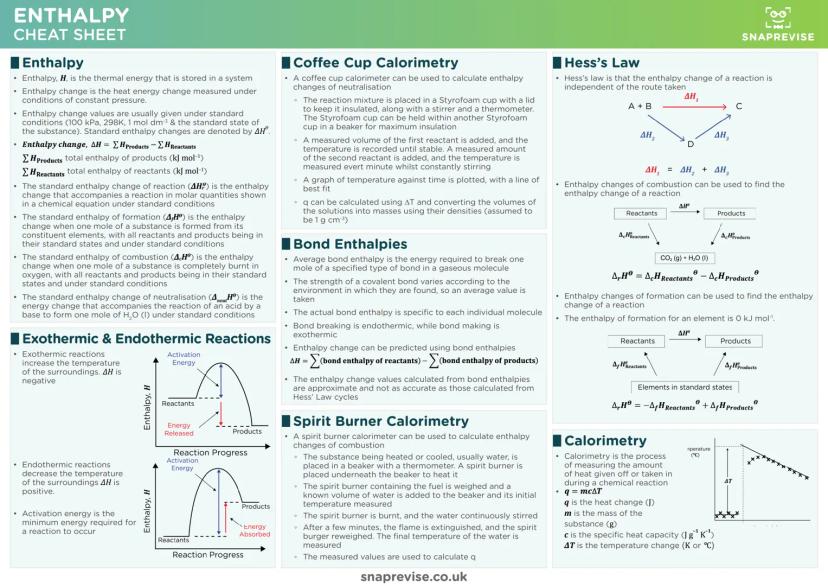
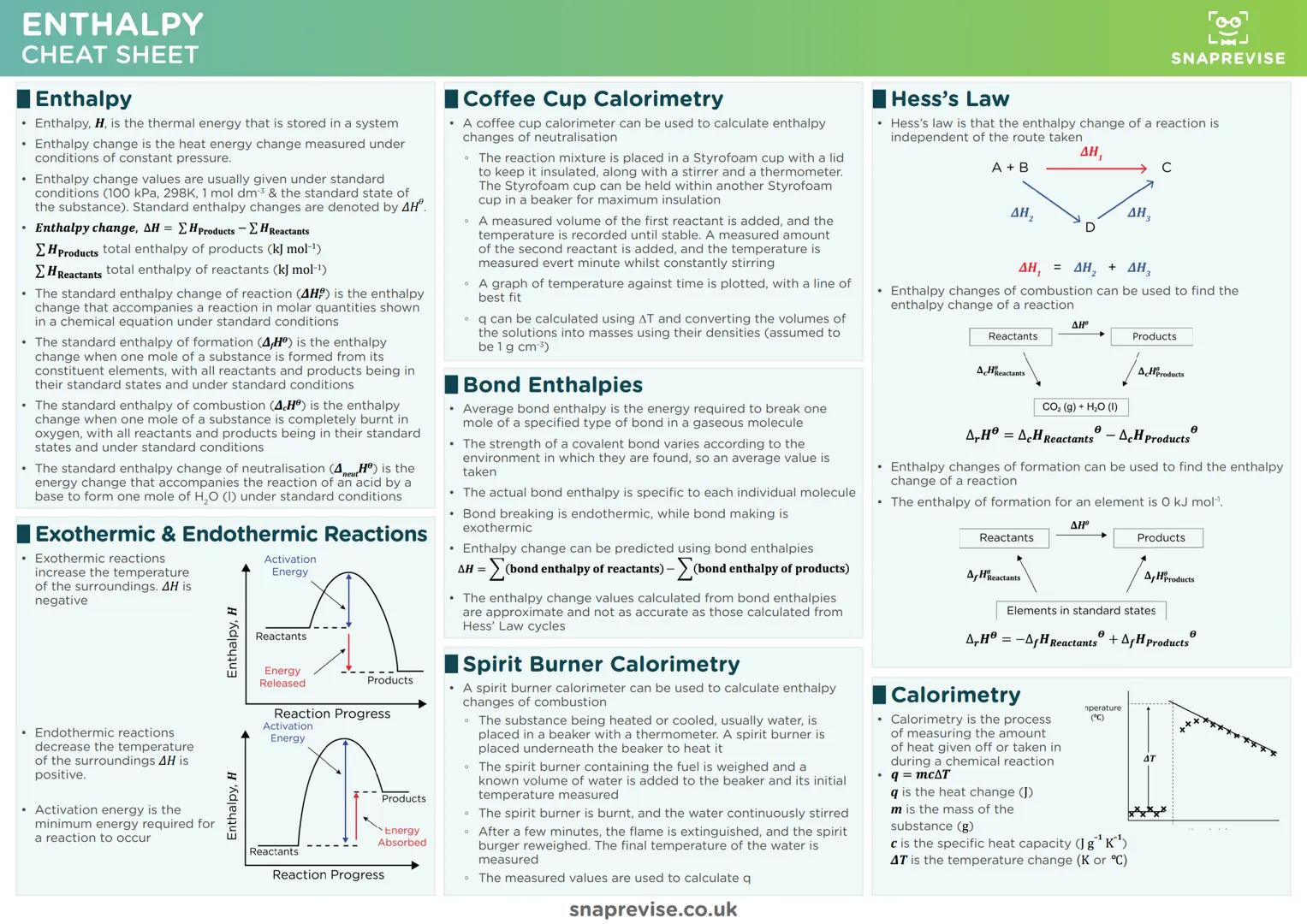
Enthalpy changes measure heat energy transfers at constant pressure, and the sign tells you everything. Negative ΔH means exothermic (energy released), positive ΔH means endothermic (energy absorbed) - think of it like your bank balance.
Standard enthalpy definitions are exam essentials. Formation enthalpy involves making one mole from elements, combustion enthalpy involves burning one mole completely, and they're all measured under standard conditions (298K, 100 kPa).
Calorimetry calculations use q = mcΔT religiously. Whether you're using coffee cup calorimeters for solution reactions or spirit burners for combustion, this equation converts temperature changes into energy values.
Practical Tip: Hess's Law cycles are like taking different routes to the same destination - the energy change is identical regardless of pathway. Use formation or combustion data to find unknown enthalpy changes.
Bond enthalpies offer another calculation route, though less accurate than Hess's Law. Remember that breaking bonds requires energy (endothermic), while making bonds releases energy (exothermic). The difference gives you ΔH.
Activation energy represents the minimum energy barrier for reactions. Catalysts don't change the overall enthalpy change - they just provide alternative routes with lower activation energies, making reactions faster.
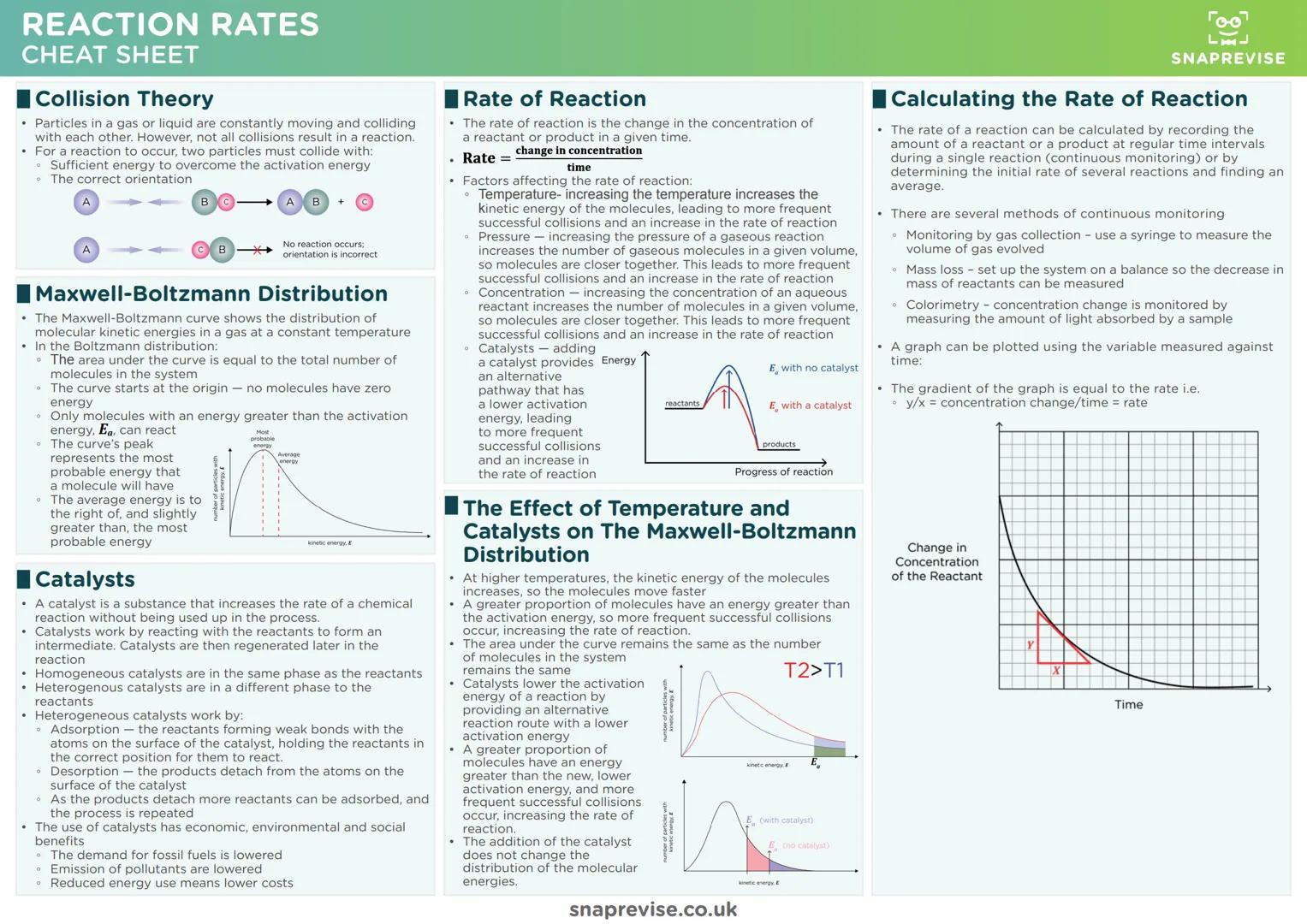
Collision theory explains why not every molecular collision leads to reaction. Particles need sufficient energy to overcome activation energy AND the correct orientation - it's like trying to fit a key in a lock while blindfolded.
The Maxwell-Boltzmann distribution shows molecular energy spread at any temperature. Only molecules with energy exceeding the activation energy can react, which explains why small temperature increases dramatically boost reaction rates.
Rate factors all work by increasing successful collision frequency. Higher temperature gives molecules more kinetic energy, increased concentration or pressure brings molecules closer together, and catalysts lower the energy barrier.
Graph Skills: When plotting concentration against time, the gradient equals reaction rate. Steeper gradients mean faster rates, and curves level off as reactants get consumed.
Heterogeneous catalysts work through adsorption and desorption cycles. Reactants stick to the catalyst surface in the right orientation, react more easily, then products leave to make space for more reactants - it's molecular matchmaking.
Temperature effects on the Maxwell-Boltzmann curve show why even small temperature rises boost rates dramatically. The curve shifts right at higher temperatures, meaning many more molecules exceed the activation energy threshold.
Organic formulae come in different flavours, each serving specific purposes. Molecular formulas just count atoms, structural formulas show connectivity, and skeletal formulas simplify everything into lines and junctions for quick drawing.
Isomerism means same molecular formula, different arrangement - like rearranging furniture in identical rooms. Structural isomers have different connectivity patterns, which can dramatically change their properties and reactions.
Nomenclature rules follow logical patterns once you crack the code. Find the longest carbon chain (stem), identify the main functional group (suffix), add any substituents (prefix), then number everything from the end giving the lowest numbers.
Drawing Tip: Skeletal formulas save massive amounts of time in exams. Lines represent C-C bonds, junctions are carbon atoms, and you only label heteroatoms (anything that's not carbon or hydrogen).
Reaction mechanisms use curly arrows to show electron movement. Homolytic fission splits bonds equally (forming radicals), while heterolytic fission gives both electrons to one atom (forming ions). The arrow tails start where electrons come from.
Functional groups determine chemical behaviour. Each group has characteristic reactions that work regardless of the rest of the molecule - once you know how alcohols behave, you can predict reactions of any alcohol.
Alcohol classification depends on how many carbon atoms connect to the carbon bearing the -OH group. This classification predicts their oxidation reactions - primary alcohols can form aldehydes then carboxylic acids, secondary alcohols form ketones, tertiary alcohols resist oxidation.
Alcohol properties reflect their ability to hydrogen bond. Short-chain alcohols dissolve readily in water, but as chain length increases, the hydrophobic alkyl portion dominates and solubility decreases dramatically.
Oxidation reactions require specific conditions for different outcomes. Gentle heating with immediate distillation converts primary alcohols to aldehydes, while heating under reflux pushes the reaction further to carboxylic acids.
Practical Point: The orange dichromate(VI) oxidising agent turns green when it gets reduced. This colour change confirms oxidation has occurred and helps identify alcohol types.
Haloalkanes undergo nucleophilic substitution because the C-X bond is polar. The δ+ carbon attracts electron-rich nucleophiles like OH⁻ ions, leading to hydrolysis reactions that swap halogens for -OH groups.
CFC ozone depletion demonstrates real-world chemistry consequences. UV radiation breaks C-Cl bonds homolytically, generating chlorine radicals that catalytically destroy ozone molecules - one chlorine radical can eliminate thousands of ozone molecules.
Synthetic routes map out how to convert one compound into another through logical reaction sequences. The flowchart shows key transformations - alkenes from dehydration, alcohols from hydration, and oxidation products from alcohols.
Mass spectrometry identifies compounds through molecular ion peaks and fragmentation patterns. The highest m/z peak gives molecular mass, while fragment patterns create unique 'fingerprints' for structural identification.
Infrared spectroscopy detects functional groups through characteristic bond vibrations. Each bond type absorbs specific frequencies - you don't need to memorise exact values, but you should recognise broad O-H stretches and sharp C=O peaks.
Analysis Strategy: Combine techniques for complete structural determination. Use molecular ion peak for molecular formula, IR for functional groups, and NMR for detailed connectivity information.
NMR spectroscopy reveals molecular environments. ¹³C NMR shows how many different carbon environments exist, while ¹H NMR gives integration ratios and splitting patterns from the n+1 rule to identify neighbouring hydrogen atoms.
The n+1 rule predicts splitting patterns in high-resolution ¹H NMR. If a hydrogen environment has n neighbouring hydrogens, its peak splits into n+1 lines - this connectivity information helps piece together molecular structures like a chemical jigsaw puzzle.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
k
@studywkay
A-level Chemistry can feel overwhelming, but this cheat sheet pack breaks down the entire OCR course into digestible chunks. You'll find all the essential definitions, key concepts, and exam-focused content that'll help you tackle everything from atomic structure to organic... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This isn't just another revision guide - it's your complete toolkit for mastering A-level Chemistry. The pack condenses two years of study into focused, exam-ready summaries that highlight what actually matters for your grades.
Every cheat sheet maps directly to OCR specification points, so you won't waste time on irrelevant details. From basic atomic structure through to complex organic mechanisms, you've got everything covered in one place.
Quick Tip: Use this pack alongside your textbook, not as a replacement. It's designed to reinforce what you've learned and fill any gaps before exams.
The content spans all six modules of the OCR course, taking you from foundational chemistry concepts right through to advanced analytical techniques. Each section builds logically on the previous one, making revision feel less chaotic and more manageable.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This pack covers every major topic you'll encounter in A-level Chemistry, from atomic structure to chromatography. The table shows exactly how each cheat sheet aligns with OCR specification points, so you know you're studying the right content.
You'll notice topics are organised logically - starting with fundamental concepts like moles and equations before building up to complex areas like transition elements and organic synthesis. This structure mirrors how chemistry concepts actually connect in real life.
Study Smart: Focus on the specification points that appear most frequently in past papers - these tend to be the bread-and-butter topics that examiners love.
The pack includes everything from Group 2 reactions to NMR spectroscopy, with each section designed to be standalone yet interconnected. Whether you're cramming for mocks or doing final revision, you can jump to any topic and find what you need quickly.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Working with ionic compounds becomes straightforward once you understand the charge balancing game. Cations lose electrons , while anions gain them, and the overall compound must be electrically neutral.
Empirical formulas give you the simplest ratio of atoms, while molecular formulas tell the full story. To find empirical formulas, convert masses to moles, then divide by the smallest number - it's like finding the lowest common denominator in maths.
The mole concept is your gateway to quantitative chemistry. Remember that one mole always contains Avogadro's number of particles (6.022 × 10²³), whether you're counting atoms, molecules, or ions. Use n = m/M religiously.
Exam Focus: Percentage yield and atom economy questions are exam favourites. Higher atom economy means less waste and better environmental impact - perfect for those evaluation questions.
Gas calculations follow predictable patterns. Under standard conditions, one mole occupies 24 dm³, and the ideal gas equation PV = nRT handles everything else. Always check your units match the gas constant value you're using.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The Periodic Table isn't random - it's organised by increasing atomic number, creating predictable patterns called periodicity. Elements in the same group share similar properties because they have identical outer shell electron configurations.
Ionisation energy trends make perfect sense when you consider three key factors: nuclear charge, atomic radius, and electron shielding. Across periods, ionisation energy increases as nuclear charge grows stronger. Down groups, it decreases as electrons get further from the nucleus.
Successive ionisation energies provide brilliant evidence for electron shell structure. You'll see gradual increases within shells, then massive jumps when electrons are removed from inner shells closer to the nucleus.
Memory Aid: Think of ionisation energy like trying to pull someone away from a crowd - the closer they are to the centre (nucleus), the harder it becomes.
Atomic radius shows clear periodicity too. Across periods, radius decreases as increasing nuclear charge pulls electrons closer. Down groups, radius increases as extra electron shells are added, despite the stronger nucleus.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Group 2 elements (alkaline earth metals) become increasingly reactive down the group because ionisation energies decrease. As atoms get larger with more shielding, it's easier to remove those outer electrons and form 2+ ions.
These metals undergo predictable redox reactions - they lose electrons and get oxidised. Whether reacting with water, oxygen, or acids, Group 2 elements always form compounds where they have a +2 oxidation state.
Group 7 elements (halogens) exist as diatomic molecules and become less reactive down the group. As atomic radius increases, it becomes harder for these atoms to attract and gain the electron needed to complete their outer shell.
Test Tip: Learn the halide precipitation tests inside out. Silver chloride is white, silver bromide is cream, silver iodide is yellow - and their different solubilities in ammonia are exam gold.
Displacement reactions prove halogen reactivity order. More reactive halogens displace less reactive halide ions from solution - chlorine kicks out bromide and iodide, bromine displaces iodide, but iodine can't displace anything.
The chlorine-water equilibrium produces both HCl and HClO through disproportionation. This reaction is crucial for water treatment, though it comes with environmental and health considerations worth memorising for evaluation questions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Enthalpy changes measure heat energy transfers at constant pressure, and the sign tells you everything. Negative ΔH means exothermic (energy released), positive ΔH means endothermic (energy absorbed) - think of it like your bank balance.
Standard enthalpy definitions are exam essentials. Formation enthalpy involves making one mole from elements, combustion enthalpy involves burning one mole completely, and they're all measured under standard conditions (298K, 100 kPa).
Calorimetry calculations use q = mcΔT religiously. Whether you're using coffee cup calorimeters for solution reactions or spirit burners for combustion, this equation converts temperature changes into energy values.
Practical Tip: Hess's Law cycles are like taking different routes to the same destination - the energy change is identical regardless of pathway. Use formation or combustion data to find unknown enthalpy changes.
Bond enthalpies offer another calculation route, though less accurate than Hess's Law. Remember that breaking bonds requires energy (endothermic), while making bonds releases energy (exothermic). The difference gives you ΔH.
Activation energy represents the minimum energy barrier for reactions. Catalysts don't change the overall enthalpy change - they just provide alternative routes with lower activation energies, making reactions faster.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Collision theory explains why not every molecular collision leads to reaction. Particles need sufficient energy to overcome activation energy AND the correct orientation - it's like trying to fit a key in a lock while blindfolded.
The Maxwell-Boltzmann distribution shows molecular energy spread at any temperature. Only molecules with energy exceeding the activation energy can react, which explains why small temperature increases dramatically boost reaction rates.
Rate factors all work by increasing successful collision frequency. Higher temperature gives molecules more kinetic energy, increased concentration or pressure brings molecules closer together, and catalysts lower the energy barrier.
Graph Skills: When plotting concentration against time, the gradient equals reaction rate. Steeper gradients mean faster rates, and curves level off as reactants get consumed.
Heterogeneous catalysts work through adsorption and desorption cycles. Reactants stick to the catalyst surface in the right orientation, react more easily, then products leave to make space for more reactants - it's molecular matchmaking.
Temperature effects on the Maxwell-Boltzmann curve show why even small temperature rises boost rates dramatically. The curve shifts right at higher temperatures, meaning many more molecules exceed the activation energy threshold.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Organic formulae come in different flavours, each serving specific purposes. Molecular formulas just count atoms, structural formulas show connectivity, and skeletal formulas simplify everything into lines and junctions for quick drawing.
Isomerism means same molecular formula, different arrangement - like rearranging furniture in identical rooms. Structural isomers have different connectivity patterns, which can dramatically change their properties and reactions.
Nomenclature rules follow logical patterns once you crack the code. Find the longest carbon chain (stem), identify the main functional group (suffix), add any substituents (prefix), then number everything from the end giving the lowest numbers.
Drawing Tip: Skeletal formulas save massive amounts of time in exams. Lines represent C-C bonds, junctions are carbon atoms, and you only label heteroatoms (anything that's not carbon or hydrogen).
Reaction mechanisms use curly arrows to show electron movement. Homolytic fission splits bonds equally (forming radicals), while heterolytic fission gives both electrons to one atom (forming ions). The arrow tails start where electrons come from.
Functional groups determine chemical behaviour. Each group has characteristic reactions that work regardless of the rest of the molecule - once you know how alcohols behave, you can predict reactions of any alcohol.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Alcohol classification depends on how many carbon atoms connect to the carbon bearing the -OH group. This classification predicts their oxidation reactions - primary alcohols can form aldehydes then carboxylic acids, secondary alcohols form ketones, tertiary alcohols resist oxidation.
Alcohol properties reflect their ability to hydrogen bond. Short-chain alcohols dissolve readily in water, but as chain length increases, the hydrophobic alkyl portion dominates and solubility decreases dramatically.
Oxidation reactions require specific conditions for different outcomes. Gentle heating with immediate distillation converts primary alcohols to aldehydes, while heating under reflux pushes the reaction further to carboxylic acids.
Practical Point: The orange dichromate(VI) oxidising agent turns green when it gets reduced. This colour change confirms oxidation has occurred and helps identify alcohol types.
Haloalkanes undergo nucleophilic substitution because the C-X bond is polar. The δ+ carbon attracts electron-rich nucleophiles like OH⁻ ions, leading to hydrolysis reactions that swap halogens for -OH groups.
CFC ozone depletion demonstrates real-world chemistry consequences. UV radiation breaks C-Cl bonds homolytically, generating chlorine radicals that catalytically destroy ozone molecules - one chlorine radical can eliminate thousands of ozone molecules.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Synthetic routes map out how to convert one compound into another through logical reaction sequences. The flowchart shows key transformations - alkenes from dehydration, alcohols from hydration, and oxidation products from alcohols.
Mass spectrometry identifies compounds through molecular ion peaks and fragmentation patterns. The highest m/z peak gives molecular mass, while fragment patterns create unique 'fingerprints' for structural identification.
Infrared spectroscopy detects functional groups through characteristic bond vibrations. Each bond type absorbs specific frequencies - you don't need to memorise exact values, but you should recognise broad O-H stretches and sharp C=O peaks.
Analysis Strategy: Combine techniques for complete structural determination. Use molecular ion peak for molecular formula, IR for functional groups, and NMR for detailed connectivity information.
NMR spectroscopy reveals molecular environments. ¹³C NMR shows how many different carbon environments exist, while ¹H NMR gives integration ratios and splitting patterns from the n+1 rule to identify neighbouring hydrogen atoms.
The n+1 rule predicts splitting patterns in high-resolution ¹H NMR. If a hydrogen environment has n neighbouring hydrogens, its peak splits into n+1 lines - this connectivity information helps piece together molecular structures like a chemical jigsaw puzzle.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
41
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user