Gas Laws and Concentrations
The ideal gas equation PV = nRT connects pressure, volume, moles, and temperature - it's like the Swiss Army knife of gas calculations. Just remember to convert everything to proper units: Kelvin for temperature, pascals for pressure, and m³ for volume.
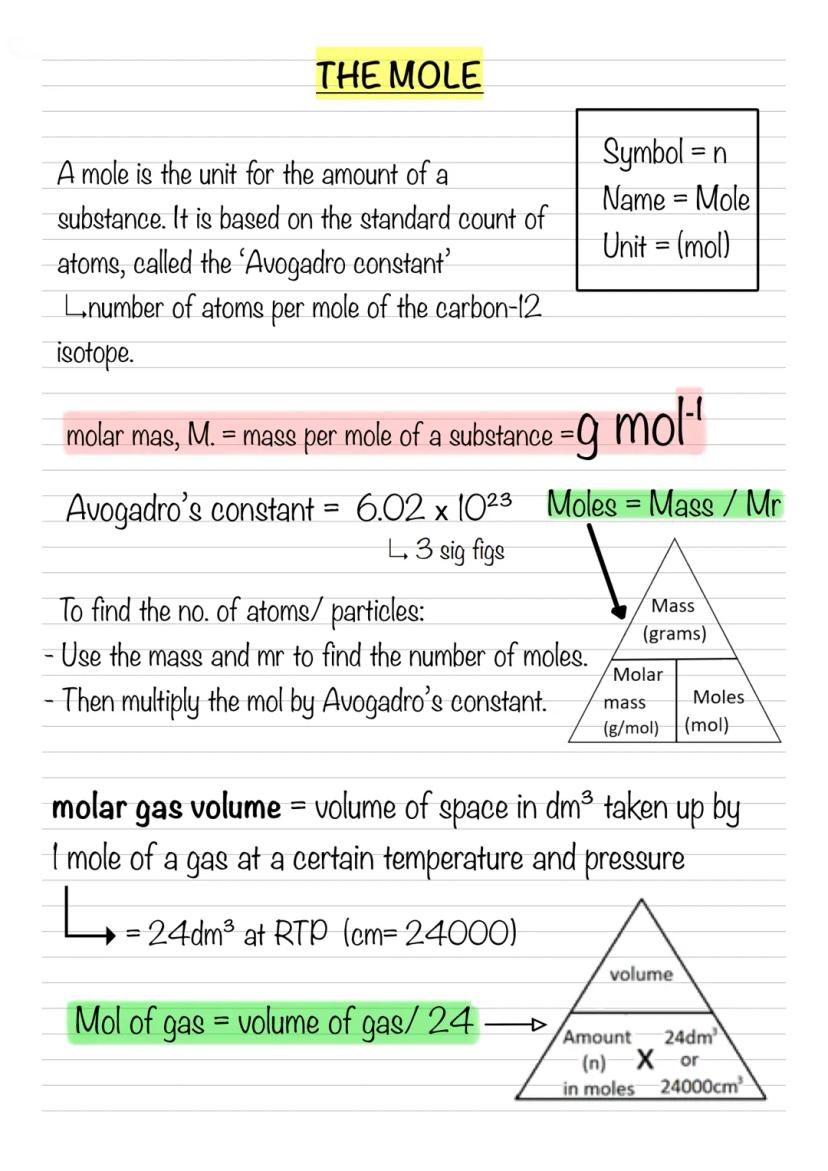
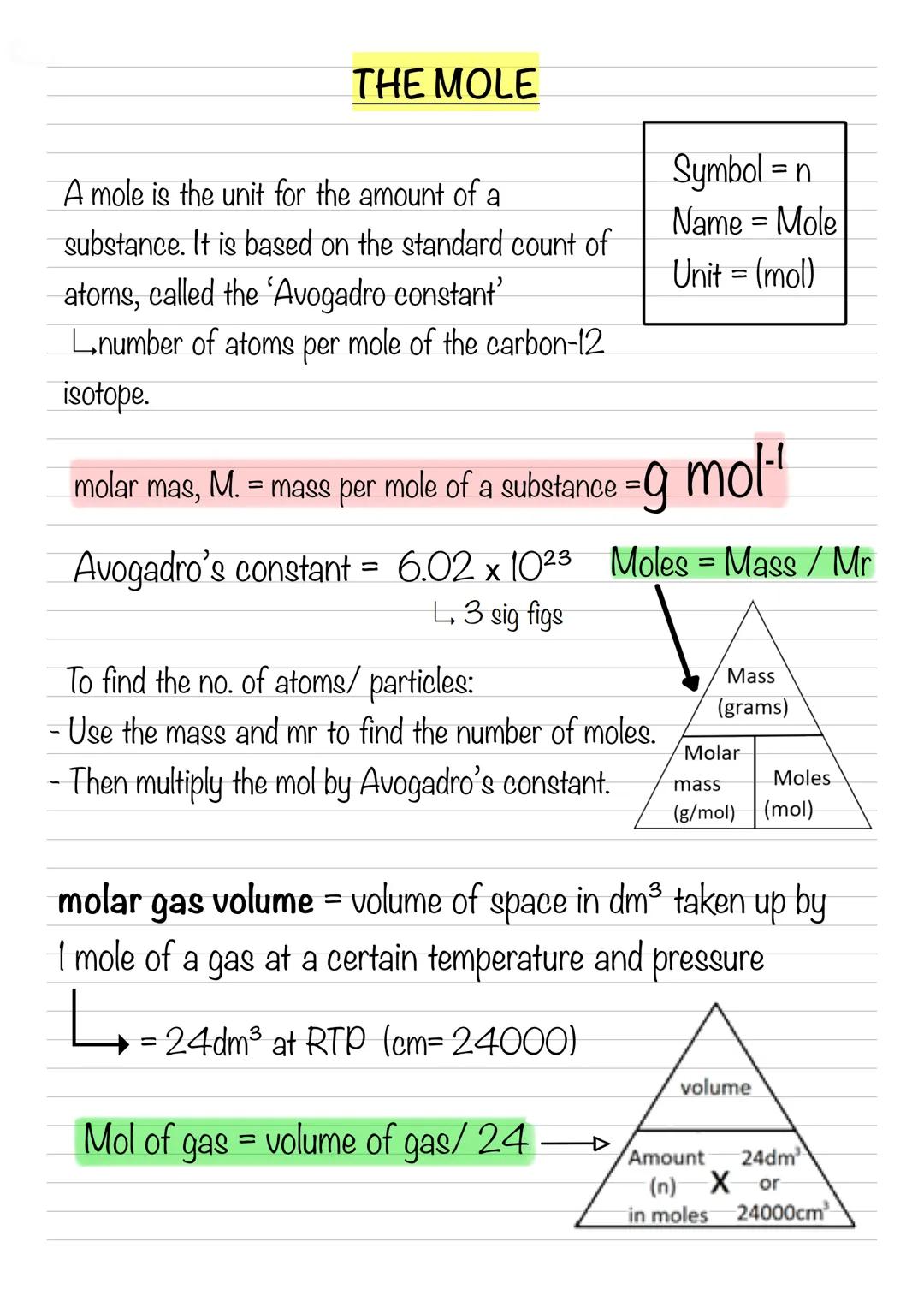
Concentration simply means how much stuff you've packed into a given volume - either as mol dm⁻³ or g dm⁻³. The formula Concentration = amount ÷ volume works for both mass and moles.
Converting units is crucial: add 273 to get Kelvin, multiply kPa by 1000 for pascals, and remember those tricky volume conversions cm3×10−6=m3. When rearranging equations, cover what you want to find and divide the rest by what's left.
Memory Aid: Think PV = nRT as "Pressure × Volume = number of moles × R × Temperature" - it reads like a sentence!