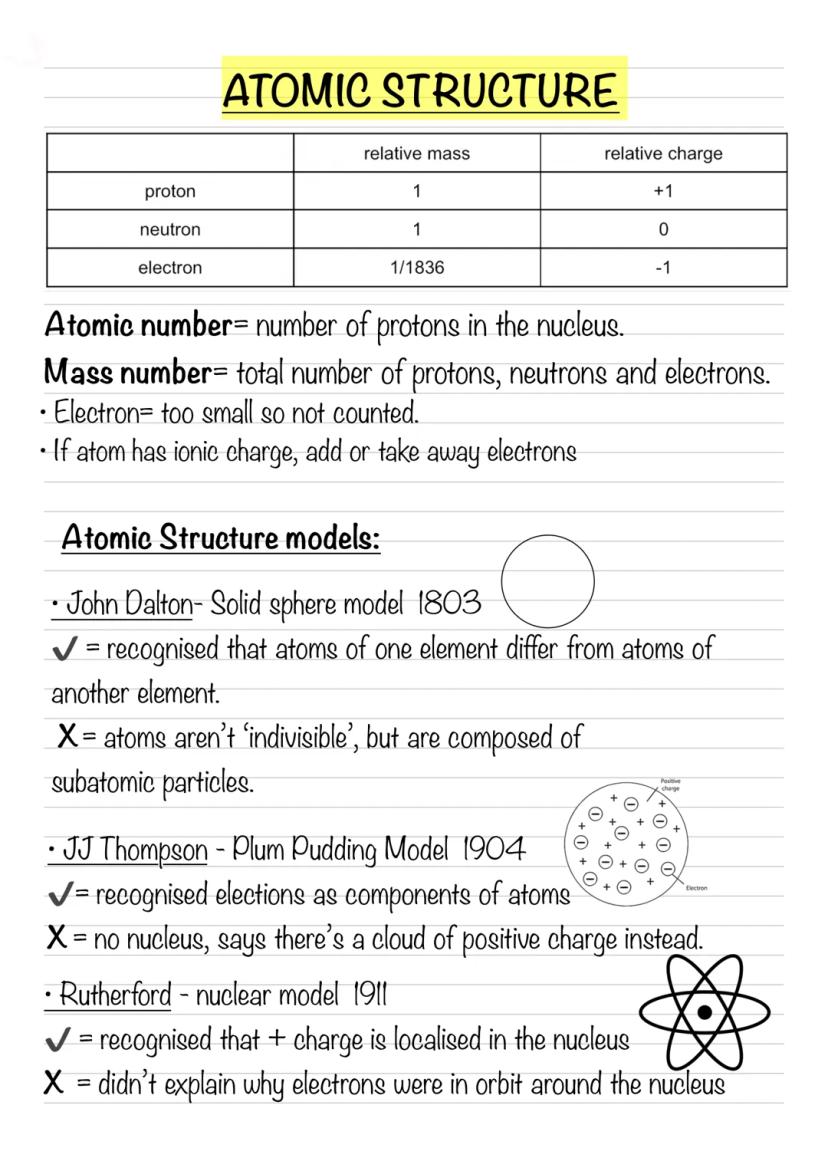
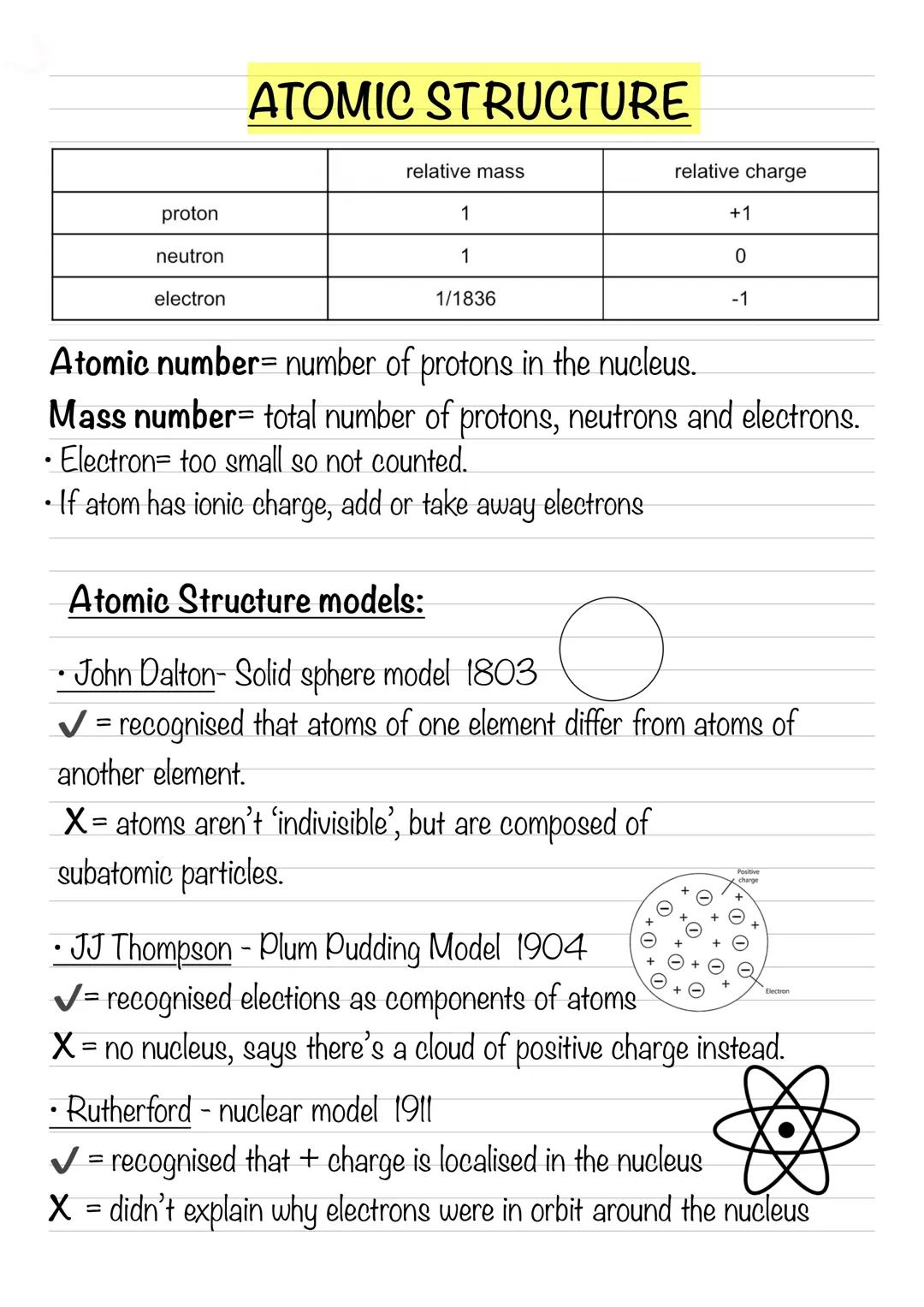
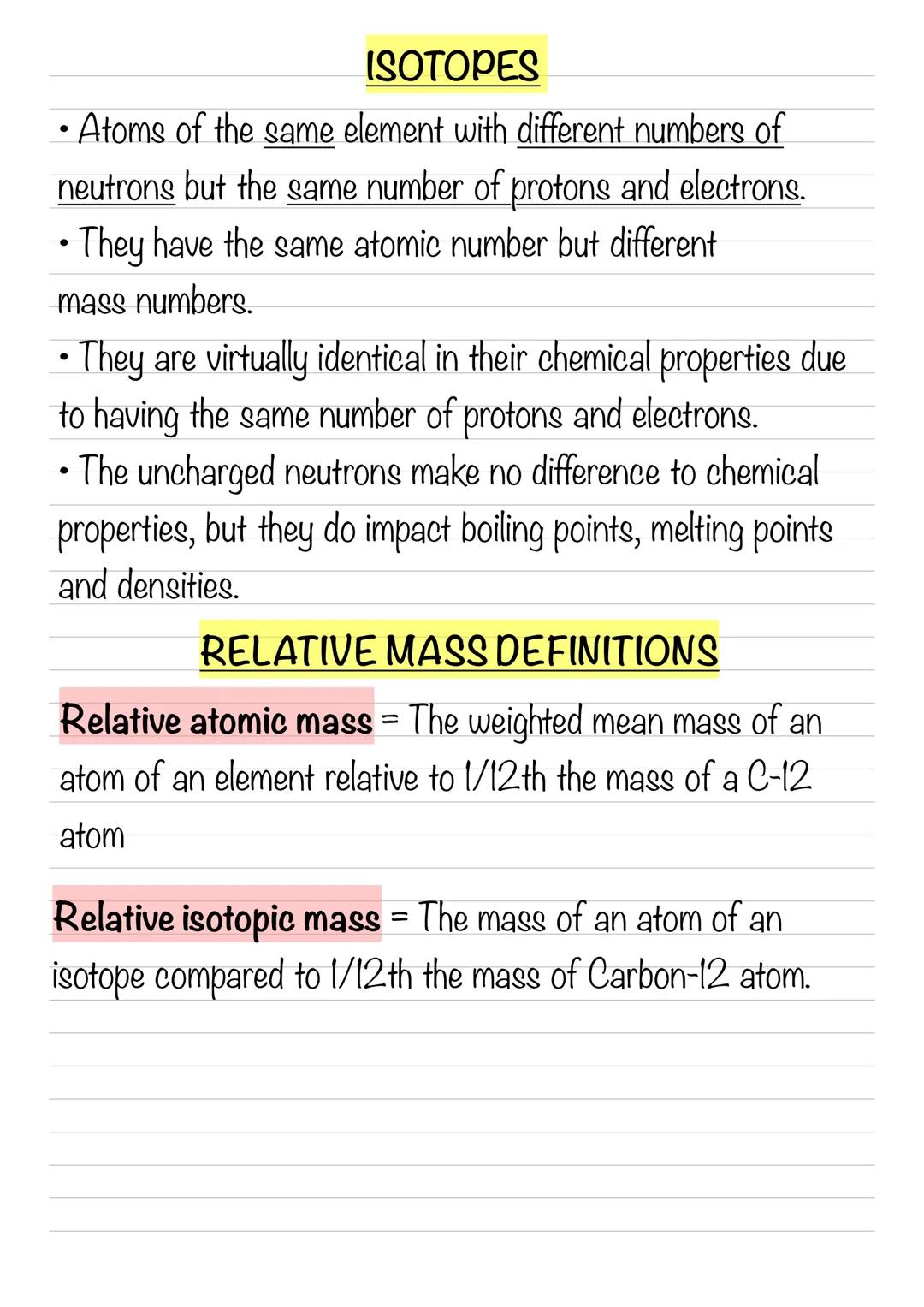
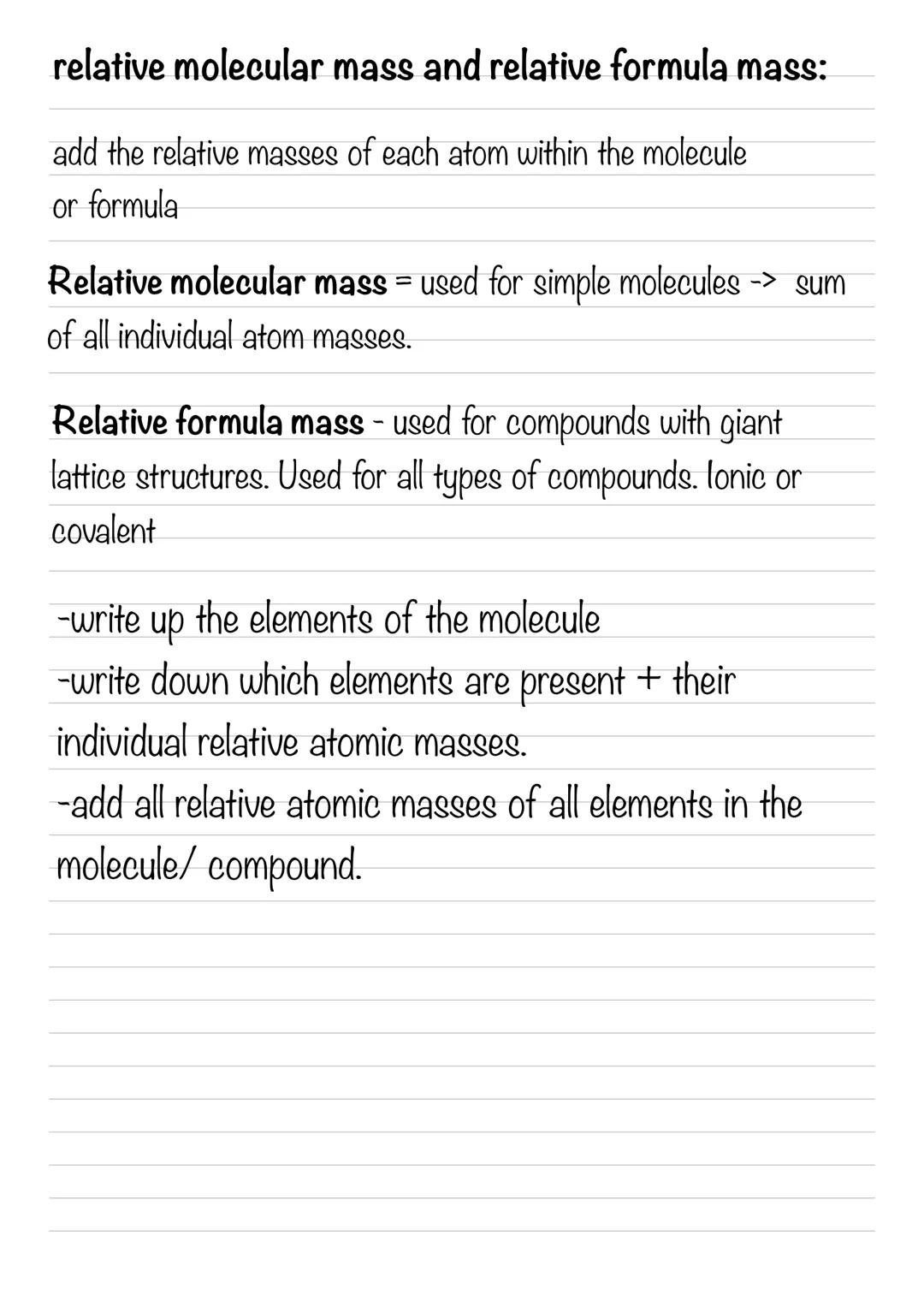
Calculating Relative Atomic Mass
You'll definitely need to master these calculations for your exams! The formula is: Ar = Σ(relative isotopic mass × abundance) ÷ 100. This weighted average accounts for how common each isotope is.
For missing isotope masses, substitute 'x' for the unknown value, expand the brackets, multiply by 100, then solve the linear equation. It's just algebra with a chemistry twist!
When calculating percentage abundances, call one isotope 'x' and the other '100-x', then follow the same process. Your final percentages must always add up to 100% - if they don't, you've made an error somewhere.
Exam Success: Always double-check your percentages add to 100% and that your final atomic mass makes sense compared to the individual isotope masses!