Ever wondered why enzymes are so crucial in your body... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
467
•
8 Feb 2026
•
Emily Anne
@emilyanne
Ever wondered why enzymes are so crucial in your body... Show more
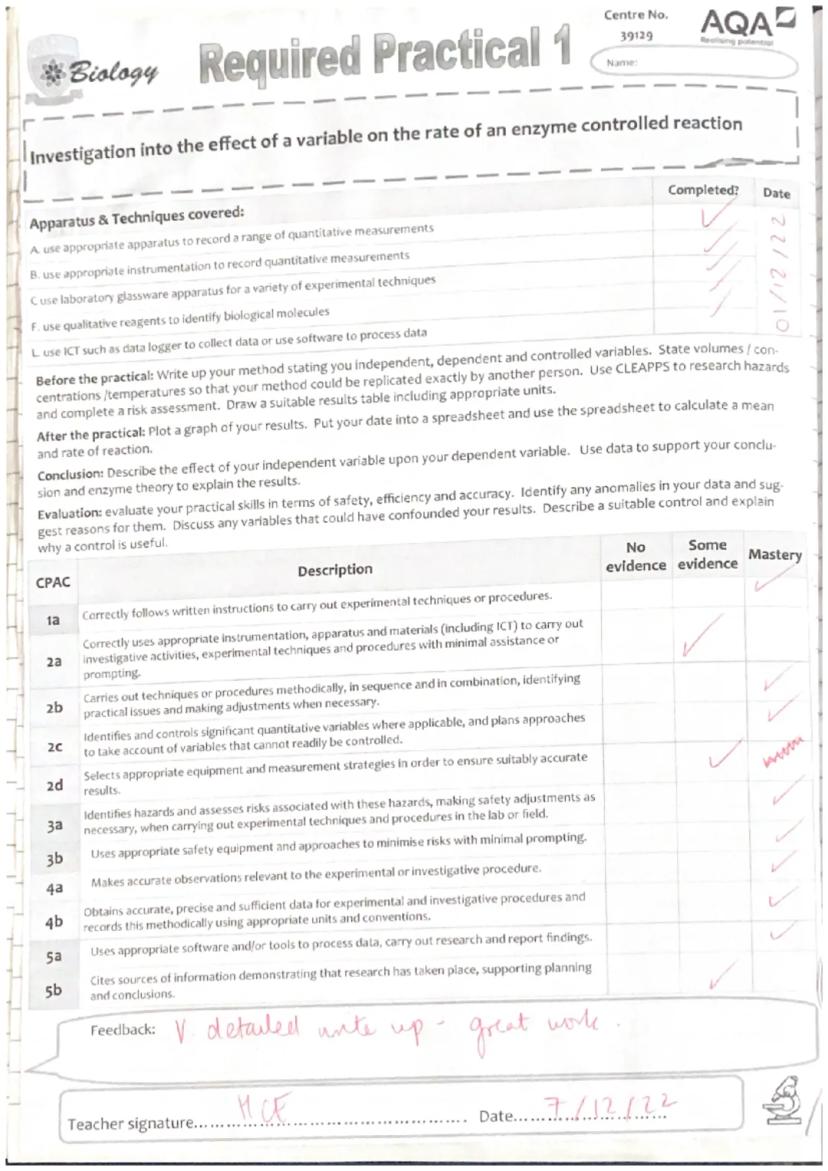





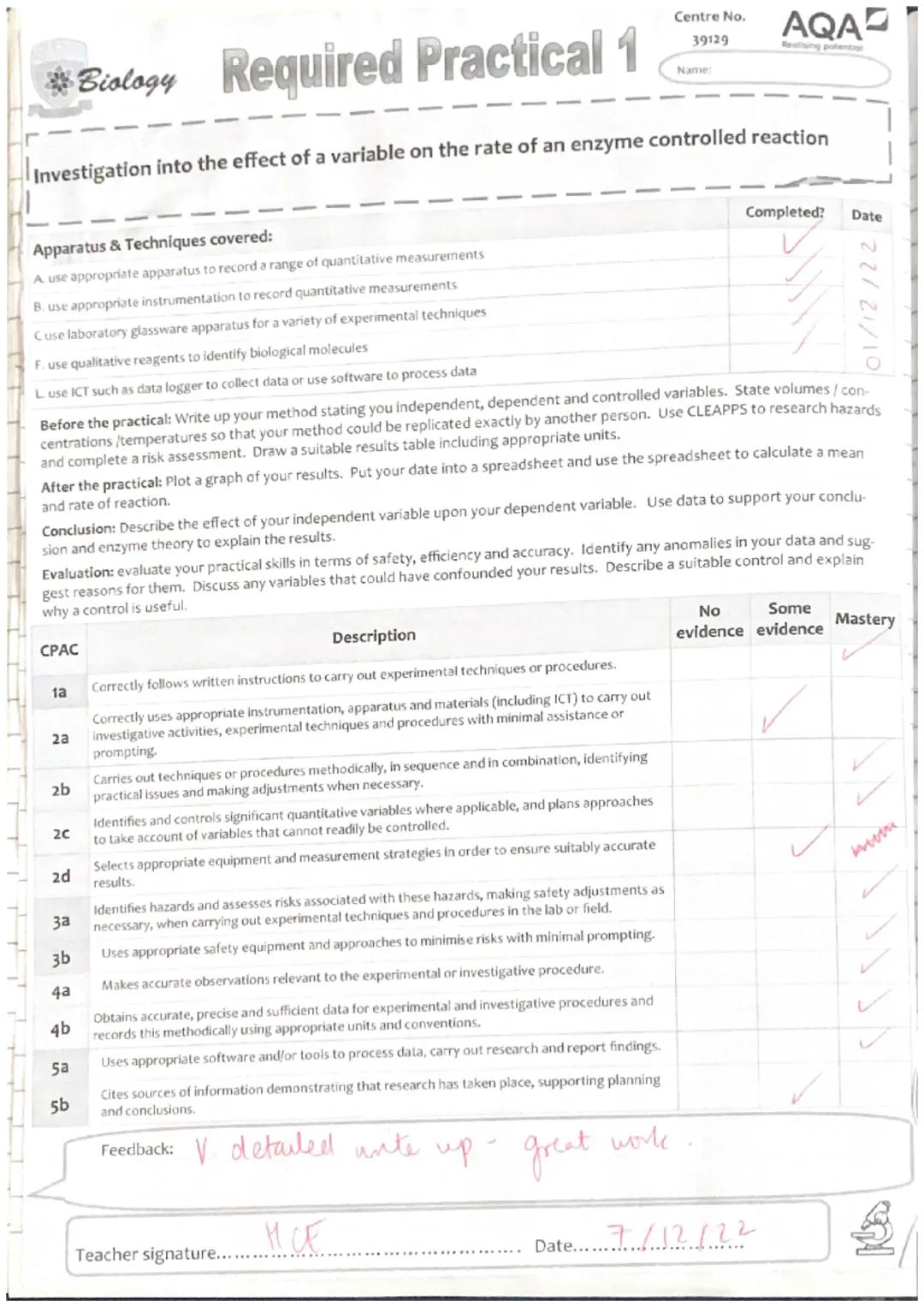
This Required Practical 1 is all about understanding how environmental factors affect enzyme activity - something that's constantly happening in your own body! You'll be investigating how temperature changes the rate at which trypsin breaks down casein protein in milk.
The practical covers loads of essential lab skills you'll need throughout A-level Biology. You'll use proper measurement techniques, handle various lab equipment safely, and learn to collect reliable data using digital tools.
Your main tasks include writing a detailed method, conducting a risk assessment, collecting data across different temperatures, and analysing your results using spreadsheets. This isn't just about following instructions - you need to think like a real scientist and justify every decision you make.
Top Tip: The key to mastering this practical is understanding that you're not just measuring times - you're exploring how molecular movement and protein structure change with temperature!

You'll be working with trypsin enzyme to break down casein protein in milk powder solution. When trypsin digests the casein, the cloudy milk solution becomes clear - that's your visual cue that the reaction is happening!
The method is straightforward but requires precision. You'll mark test tubes with an 'X', add specific volumes of milk powder solution, then mix in trypsin with pH buffer at three different temperatures (including 60°C and room temperature).
Timing is everything - you'll measure how long it takes to see the 'X' through the clearing solution. The faster you can see through it, the quicker the enzyme is working.
You'll need to control your water bath temperatures carefully and think about how to maintain room temperature conditions. Remember, you're testing at least three different temperatures to see the full picture of how temperature affects enzyme activity.
Key Point: The clearer the solution gets, the more protein has been broken down - this is your measure of enzyme activity!

Getting your variables sorted is absolutely crucial for reliable results. Your independent variable is temperature, your dependent variable is the time taken to see the 'X' through the solution, and you've got several important controlled variables to manage.
You must keep the trypsin concentration at 0.5%, milk powder at 3%, and pH constant using buffer solution. If any of these change between tests, your results won't show the true effect of temperature alone.
Temperature control requires constant monitoring with a digital thermometer. You'll need to add hot or cold water to maintain your target temperatures and record temperatures at the start and end of each reaction period.
For room temperature experiments, you'll need to minimise air movement and temperature fluctuations by controlling doors and windows. Any temperature changes during the reaction could throw off your results completely.
Remember: Every variable you don't control could be the reason your results don't match the textbook theory!

Laboratory safety isn't just about ticking boxes - it's about protecting yourself and getting reliable results. You'll be working with hot water baths (burns risk), glassware (breakage), and various chemical solutions.
The main hazards include potential burns from 60°C water baths, allergic reactions to milk powder or trypsin, and skin irritation from buffer solutions. Always use protective equipment like goggles and handle hot test tubes with tongs or cloths.
Spillages are your biggest day-to-day risk - they create slip hazards and waste time. Clean up immediately with blue roll and inform your teacher. If thermometers break, don't try to clean up glass yourself.
The trypsin solution can be corrosive and cause allergies, whilst the pH buffer is an irritant. Never ingest any solutions, wash off spillages immediately, and wear appropriate protection throughout the practical.
Safety Tip: Most accidents happen when you're rushing - take your time and follow every safety step, even if it seems obvious!
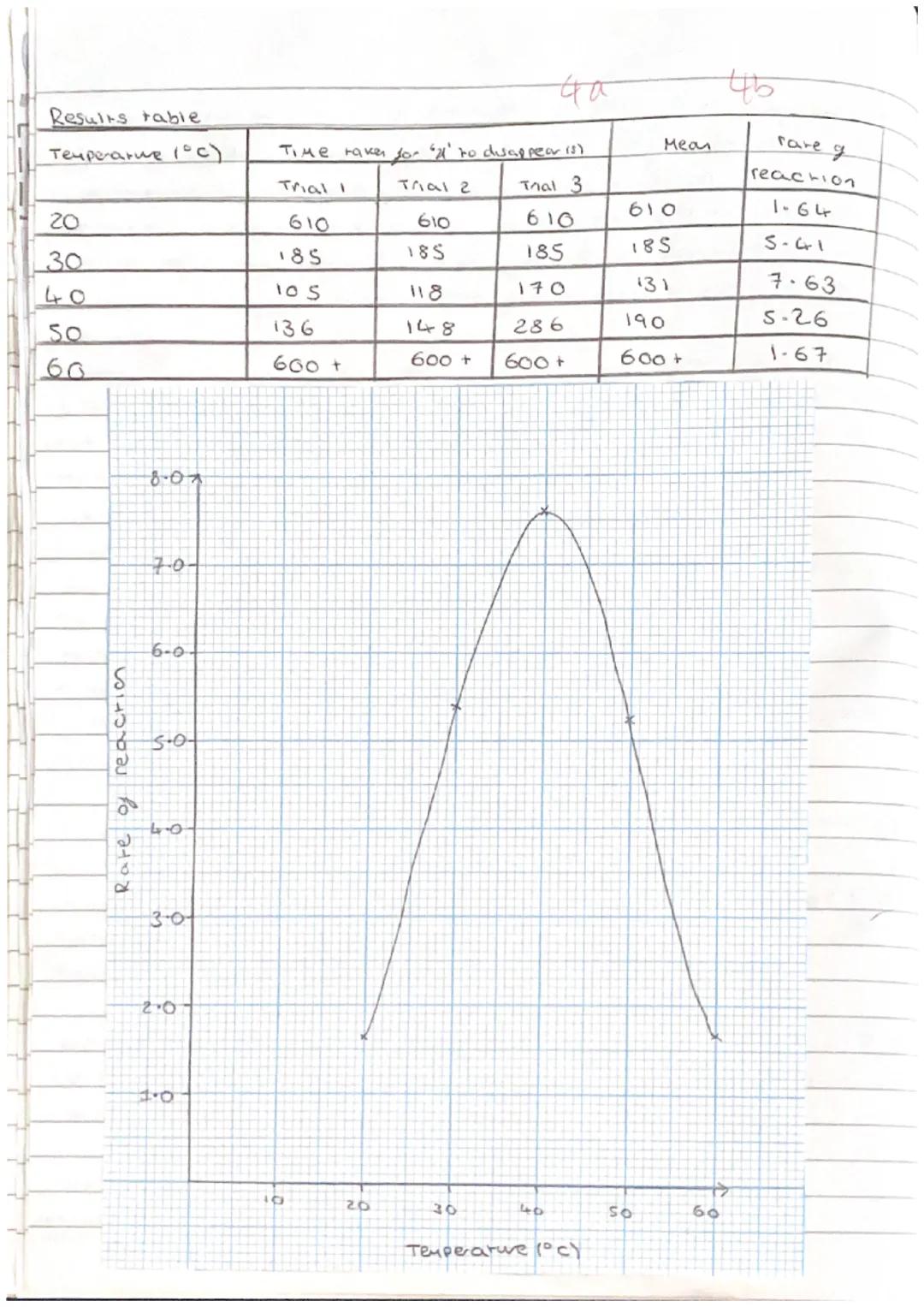
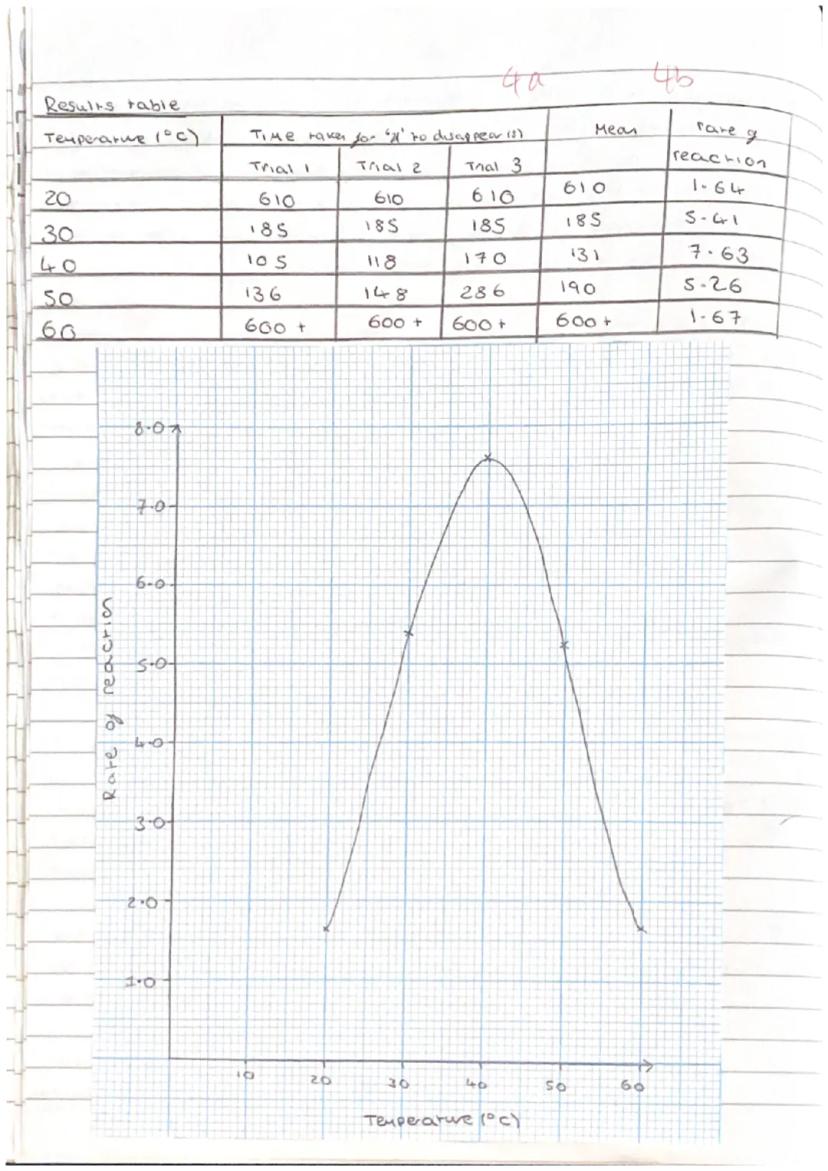
Your results table should show a clear pattern - reaction rates increasing with temperature up to an optimum point, then decreasing as enzymes denature. Typical results show fastest reactions around 40°C with slower rates at both lower and higher temperatures.
Calculating rate of reaction means dividing 1 by the time taken . This gives you positive values that increase as reactions speed up, making your graphs easier to interpret.
Your graph should show the classic enzyme-temperature relationship: low activity at room temperature, peak activity at the optimum temperature, then declining activity as temperature increases further.
Data analysis involves calculating means, identifying anomalies, and using spreadsheet software to process your results professionally. Any results that don't fit the pattern need investigating and explaining.
Graph Tip: Your rate vs temperature graph should look like a hill - climbing up to the optimum, then falling off as enzymes denature!

The enzyme-temperature relationship you've discovered follows predictable molecular principles. At low temperatures, enzymes have less kinetic energy, so fewer enzyme-substrate complexes form, resulting in slower reactions.
As temperature increases towards 40°C, molecules move faster, collision frequency increases, and more successful enzyme-substrate interactions occur. This is why your rate of reaction peaks around this temperature - it's the optimum for trypsin activity.
Above the optimum temperature, enzyme proteins begin to denature. The tertiary structure breaks down, changing the active site shape so it's no longer complementary to the substrate. Fewer enzyme-substrate complexes can form, so reaction rates decrease.
Your practical demonstrates that enzyme activity is incredibly sensitive to environmental conditions. This same principle explains why your body maintains such precise temperature control and why fever can be dangerous.
Key Insight: Enzymes are like Goldilocks - they need conditions that are 'just right', not too hot, not too cold!

Accuracy improvements could include using a colorimeter instead of visual observation. This would give quantitative rather than qualitative data, removing human subjectivity from colour change assessment.
Having one person consistently judge the endpoint helped maintain reliability, but instrumental measurement would be even better. Consistent volumes and careful variable control ensured temperature was truly the only factor being investigated.
The practical's main limitation is the subjective nature of determining when the solution becomes clear enough to see the 'X'. Different people might judge this endpoint differently, affecting result consistency.
Future modifications might include automated monitoring systems, more precise temperature control, or investigating additional temperatures to map the enzyme activity curve more completely. Understanding these limitations helps you become a better scientist.
Evaluation Focus: Great scientists always ask 'How could this be done better?' - that's how scientific knowledge advances!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Emily Anne
@emilyanne
Ever wondered why enzymes are so crucial in your body and how they actually work? This practical investigation explores how temperature affects enzyme activity using trypsin (a protein-digesting enzyme) and milk protein, giving you hands-on experience with one of biology's... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
This Required Practical 1 is all about understanding how environmental factors affect enzyme activity - something that's constantly happening in your own body! You'll be investigating how temperature changes the rate at which trypsin breaks down casein protein in milk.
The practical covers loads of essential lab skills you'll need throughout A-level Biology. You'll use proper measurement techniques, handle various lab equipment safely, and learn to collect reliable data using digital tools.
Your main tasks include writing a detailed method, conducting a risk assessment, collecting data across different temperatures, and analysing your results using spreadsheets. This isn't just about following instructions - you need to think like a real scientist and justify every decision you make.
Top Tip: The key to mastering this practical is understanding that you're not just measuring times - you're exploring how molecular movement and protein structure change with temperature!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
You'll be working with trypsin enzyme to break down casein protein in milk powder solution. When trypsin digests the casein, the cloudy milk solution becomes clear - that's your visual cue that the reaction is happening!
The method is straightforward but requires precision. You'll mark test tubes with an 'X', add specific volumes of milk powder solution, then mix in trypsin with pH buffer at three different temperatures (including 60°C and room temperature).
Timing is everything - you'll measure how long it takes to see the 'X' through the clearing solution. The faster you can see through it, the quicker the enzyme is working.
You'll need to control your water bath temperatures carefully and think about how to maintain room temperature conditions. Remember, you're testing at least three different temperatures to see the full picture of how temperature affects enzyme activity.
Key Point: The clearer the solution gets, the more protein has been broken down - this is your measure of enzyme activity!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Getting your variables sorted is absolutely crucial for reliable results. Your independent variable is temperature, your dependent variable is the time taken to see the 'X' through the solution, and you've got several important controlled variables to manage.
You must keep the trypsin concentration at 0.5%, milk powder at 3%, and pH constant using buffer solution. If any of these change between tests, your results won't show the true effect of temperature alone.
Temperature control requires constant monitoring with a digital thermometer. You'll need to add hot or cold water to maintain your target temperatures and record temperatures at the start and end of each reaction period.
For room temperature experiments, you'll need to minimise air movement and temperature fluctuations by controlling doors and windows. Any temperature changes during the reaction could throw off your results completely.
Remember: Every variable you don't control could be the reason your results don't match the textbook theory!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Laboratory safety isn't just about ticking boxes - it's about protecting yourself and getting reliable results. You'll be working with hot water baths (burns risk), glassware (breakage), and various chemical solutions.
The main hazards include potential burns from 60°C water baths, allergic reactions to milk powder or trypsin, and skin irritation from buffer solutions. Always use protective equipment like goggles and handle hot test tubes with tongs or cloths.
Spillages are your biggest day-to-day risk - they create slip hazards and waste time. Clean up immediately with blue roll and inform your teacher. If thermometers break, don't try to clean up glass yourself.
The trypsin solution can be corrosive and cause allergies, whilst the pH buffer is an irritant. Never ingest any solutions, wash off spillages immediately, and wear appropriate protection throughout the practical.
Safety Tip: Most accidents happen when you're rushing - take your time and follow every safety step, even if it seems obvious!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Your results table should show a clear pattern - reaction rates increasing with temperature up to an optimum point, then decreasing as enzymes denature. Typical results show fastest reactions around 40°C with slower rates at both lower and higher temperatures.
Calculating rate of reaction means dividing 1 by the time taken . This gives you positive values that increase as reactions speed up, making your graphs easier to interpret.
Your graph should show the classic enzyme-temperature relationship: low activity at room temperature, peak activity at the optimum temperature, then declining activity as temperature increases further.
Data analysis involves calculating means, identifying anomalies, and using spreadsheet software to process your results professionally. Any results that don't fit the pattern need investigating and explaining.
Graph Tip: Your rate vs temperature graph should look like a hill - climbing up to the optimum, then falling off as enzymes denature!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The enzyme-temperature relationship you've discovered follows predictable molecular principles. At low temperatures, enzymes have less kinetic energy, so fewer enzyme-substrate complexes form, resulting in slower reactions.
As temperature increases towards 40°C, molecules move faster, collision frequency increases, and more successful enzyme-substrate interactions occur. This is why your rate of reaction peaks around this temperature - it's the optimum for trypsin activity.
Above the optimum temperature, enzyme proteins begin to denature. The tertiary structure breaks down, changing the active site shape so it's no longer complementary to the substrate. Fewer enzyme-substrate complexes can form, so reaction rates decrease.
Your practical demonstrates that enzyme activity is incredibly sensitive to environmental conditions. This same principle explains why your body maintains such precise temperature control and why fever can be dangerous.
Key Insight: Enzymes are like Goldilocks - they need conditions that are 'just right', not too hot, not too cold!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Accuracy improvements could include using a colorimeter instead of visual observation. This would give quantitative rather than qualitative data, removing human subjectivity from colour change assessment.
Having one person consistently judge the endpoint helped maintain reliability, but instrumental measurement would be even better. Consistent volumes and careful variable control ensured temperature was truly the only factor being investigated.
The practical's main limitation is the subjective nature of determining when the solution becomes clear enough to see the 'X'. Different people might judge this endpoint differently, affecting result consistency.
Future modifications might include automated monitoring systems, more precise temperature control, or investigating additional temperatures to map the enzyme activity curve more completely. Understanding these limitations helps you become a better scientist.
Evaluation Focus: Great scientists always ask 'How could this be done better?' - that's how scientific knowledge advances!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
9
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the Krebs Cycle and Link Reaction in cellular respiration. This summary covers key processes including dehydrogenation, decarboxylation, and the formation of NADH and FADH2. Understand how Acetyl CoA interacts with Oxaloacetate to produce ATP and carbon dioxide. Ideal for A-Level Biology students studying WJEC Unit 3.
Explore the key processes of cellular respiration, including glycolysis, the Krebs cycle, and oxidative phosphorylation. This summary highlights ATP production, metabolic pathways, and the role of NADH and FADH2 in energy transfer. Ideal for A2 Level WJEC biology students.
Explore the intricate processes of photosynthesis, focusing on the light-dependent and light-independent reactions. This summary covers key concepts such as chloroplast structure, the role of chlorophyll, photolysis, ATP production, and the Calvin cycle. Ideal for A Level Biology students seeking a comprehensive understanding of photosynthesis.
Explore the intricate processes of photosynthesis, including light-dependent and light-independent reactions, chloroplast structure, and the role of pigments like chlorophyll. This summary covers key concepts such as thylakoids, the Calvin cycle, and the action spectrum, providing a comprehensive overview for A-level Biology students.
Explore the process of chemiosmosis in photosynthesis, detailing the light-dependent reactions that occur in the thylakoid membranes. This study note covers the electron transport chain, ATP synthesis via ATP synthase, and the role of NADPH. Ideal for AQA Biology students preparing for exams.
Explore the Calvin Cycle, the light-independent reactions of photosynthesis occurring in the stroma. This summary covers the journey of carbon dioxide, the role of RuBISCO, ATP and NADPH in synthesizing glucose, and the regeneration of RuBP. Ideal for students studying photosynthesis and its biochemical processes.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user