Get ready to master the essential chemistry topics you'll need... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
166
•
3 Feb 2026
•
oliviaa2008
@oliviaa2008
Get ready to master the essential chemistry topics you'll need... Show more



Ever wondered how plastics are made or why ethanol burns so well? Organic chemistry is all about carbon-based compounds that form the building blocks of everything from fuels to medicines. You'll find these molecules everywhere in daily life.
Alcohols like ethanol have the functional group -OH and follow the formula C<sub>n</sub>H<sub>2n+1</sub>OH. They're flammable, soluble in water, and can be oxidised to form carboxylic acids. Ethanol is particularly important - it's used in alcoholic drinks, as biofuel, and as a chemical feedstock for making other compounds.
Alkanes are saturated hydrocarbons that get more viscous and less flammable as their chain length increases. Through cracking (thermal decomposition), we can break long alkanes into shorter, more useful alkanes and alkenes. This process uses either steam cracking or catalytic cracking with aluminium oxide.
Addition polymers form when alkene monomers join together after their double bonds split. Meanwhile, condensation polymers like polyesters form when monomers with at least two functional groups join, producing water as a byproduct.
Key Tip: Remember that alkenes are more reactive than alkanes because of their double bonds - they'll turn bromine water from orange to colourless!
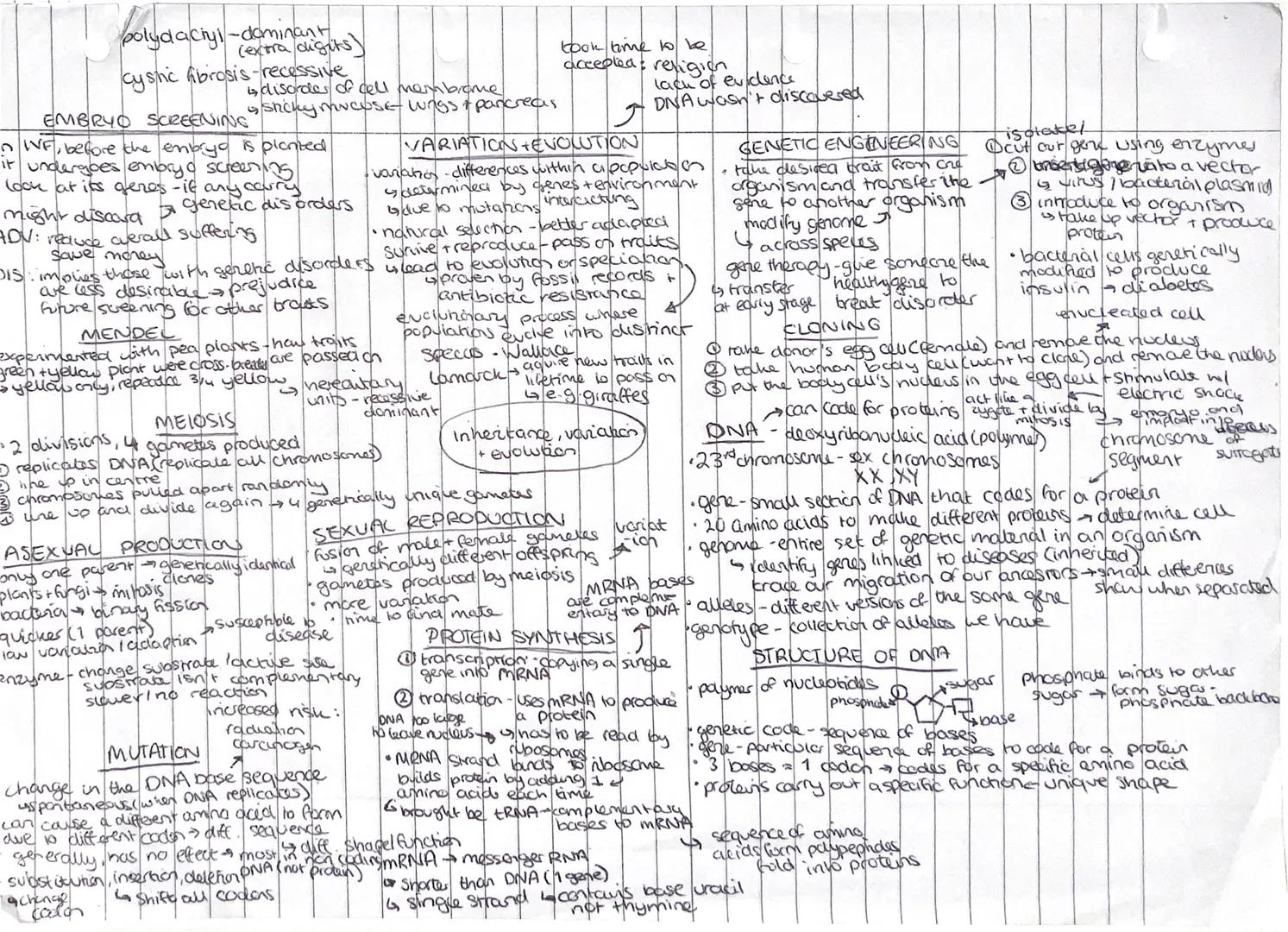
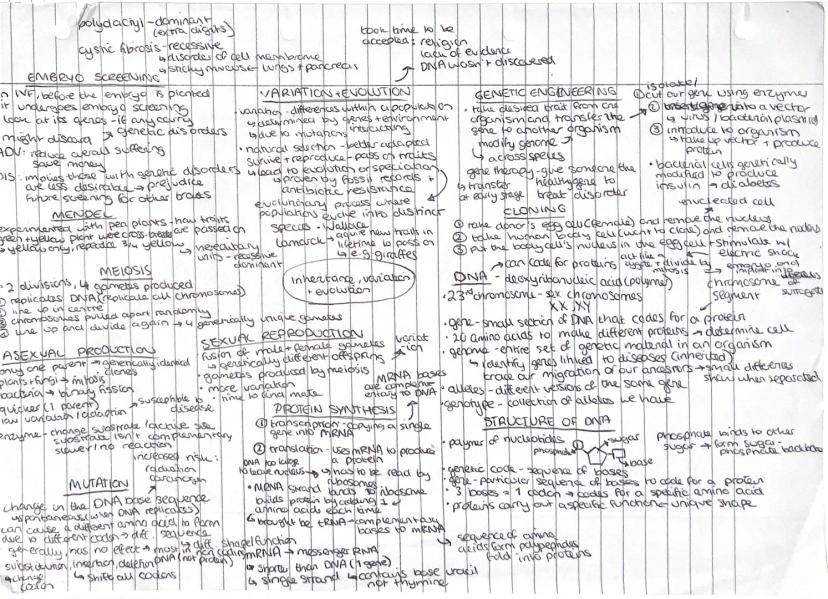
Your DNA holds the instruction manual for making you who you are! Genetic engineering allows scientists to cut genes from one organism and transfer them to another, creating everything from insulin for diabetics to disease-resistant crops.
DNA is a polymer made of nucleotides, each containing a phosphate, sugar, and base. The genetic code uses sequences of three bases (codons) to specify which amino acid gets added during protein synthesis. This happens through transcription (copying DNA to mRNA) and translation (using mRNA to build proteins).
Variation within populations comes from genes and environment interacting. Natural selection means better-adapted organisms survive and reproduce, passing on favourable traits. This leads to evolution - a process proven by fossil records and observable in antibiotic resistance.
Meiosis produces four genetically unique gametes through two divisions, whilst sexual reproduction creates genetically different offspring. Asexual reproduction is quicker but produces identical clones with no variation.
Key Tip: Mutations in DNA can change amino acid sequences, potentially altering protein function - but most mutations actually have no effect!
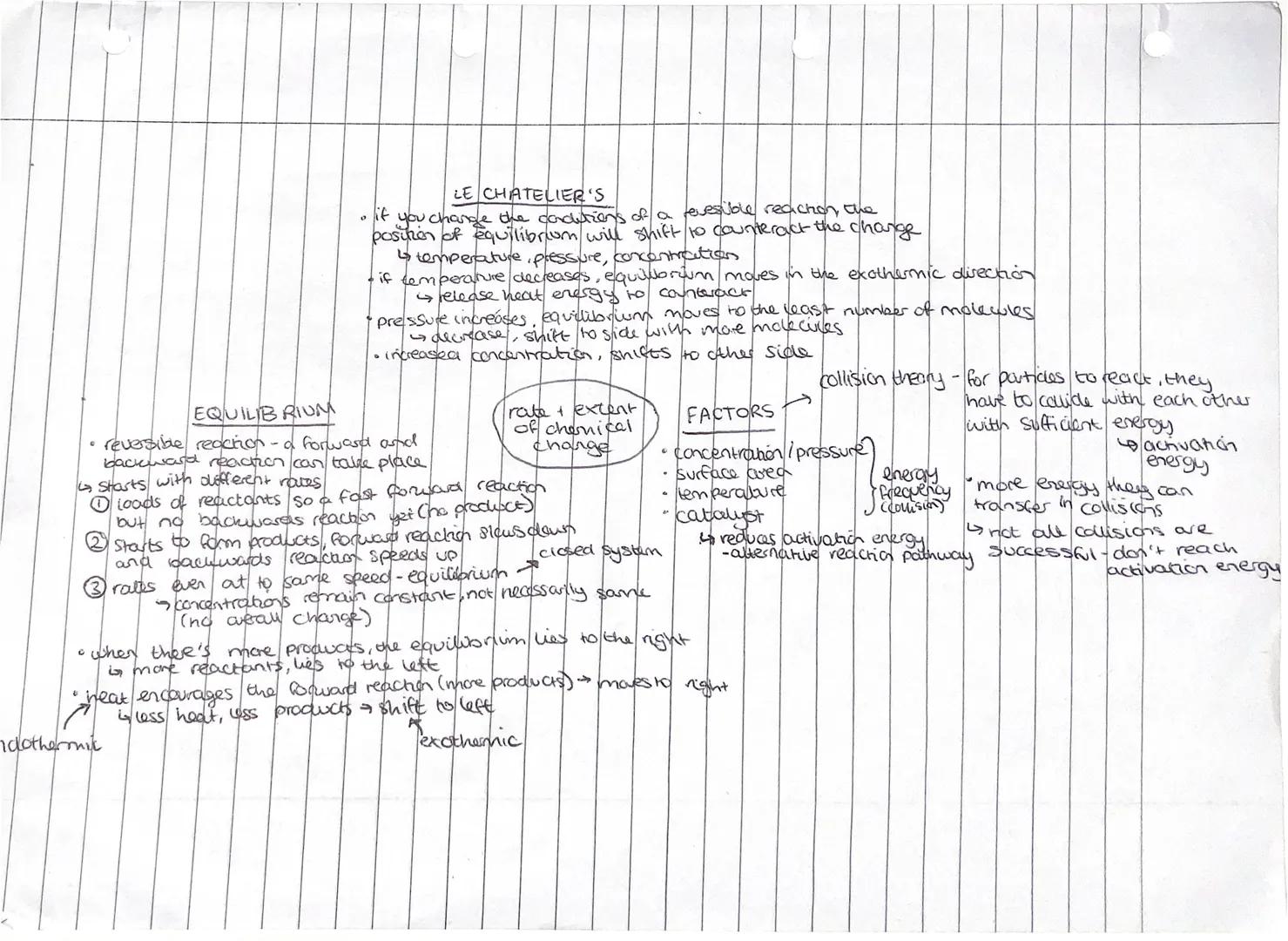
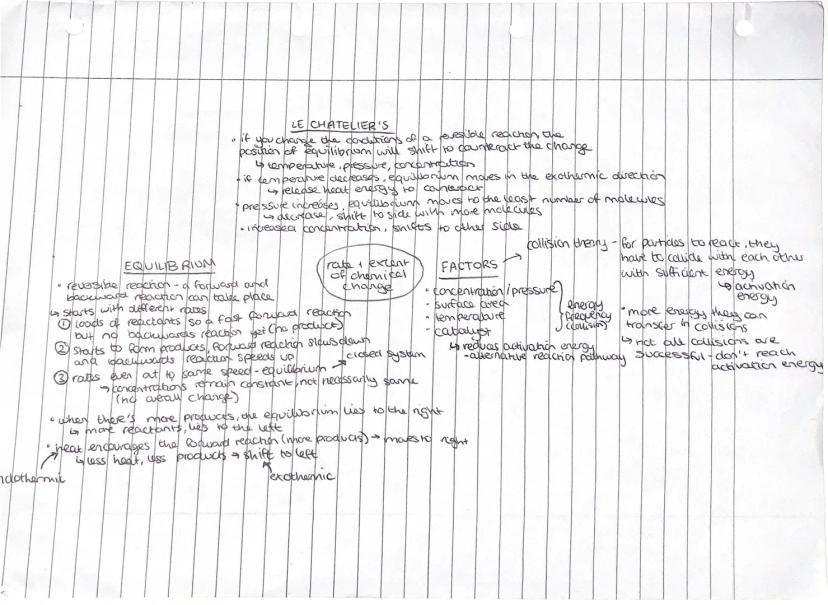
Want to control chemical reactions like a pro? Le Chatelier's Principle is your secret weapon - it predicts how equilibrium shifts when you change temperature, pressure, or concentration.
Reversible reactions reach equilibrium when forward and backward reaction rates become equal. Initially, you have loads of reactants and a fast forward reaction. As products form, the forward reaction slows whilst the backward reaction speeds up until they balance out.
Temperature changes shift equilibrium towards the reaction that counteracts the change. If you increase temperature, equilibrium moves towards the endothermic direction to absorb the extra heat. Pressure increases favour the side with fewer gas molecules.
Collision theory explains reaction rates - particles must collide with sufficient activation energy to react. You can speed up reactions by increasing concentration, pressure, surface area, temperature, or adding a catalyst. Catalysts provide alternative reaction pathways with lower activation energy.
Key Tip: At equilibrium, concentrations stay constant but aren't necessarily equal - there could be more products than reactants or vice versa!

Testing for different ions and substances is like being a chemical detective! Flame tests give specific colours for metal ions - lithium burns crimson, calcium gives orange-red, whilst sodium produces a bright yellow flame.
For anion tests, add dilute HCl to carbonates and pass the gas through limewater (turns cloudy). Sulfate ions form white precipitates with barium chloride, whilst halide ions create different coloured precipitates with silver nitrate - chloride gives white, bromide gives cream, and iodide gives yellow.
Flame emission spectroscopy is more accurate than simple flame tests because it detects specific wavelengths of light emitted by heated metal ions. Each metal produces a unique spectrum pattern, and light intensity indicates concentration.
Chromatography separates mixtures by dissolving substances in a solvent. More soluble substances travel further up the paper. Pure substances melt and boil at specific temperatures, whilst impure substances melt and boil over temperature ranges.
Key Tip: Always use pencil for chromatography baselines because ink would interfere with your results by dissolving in the solvent!
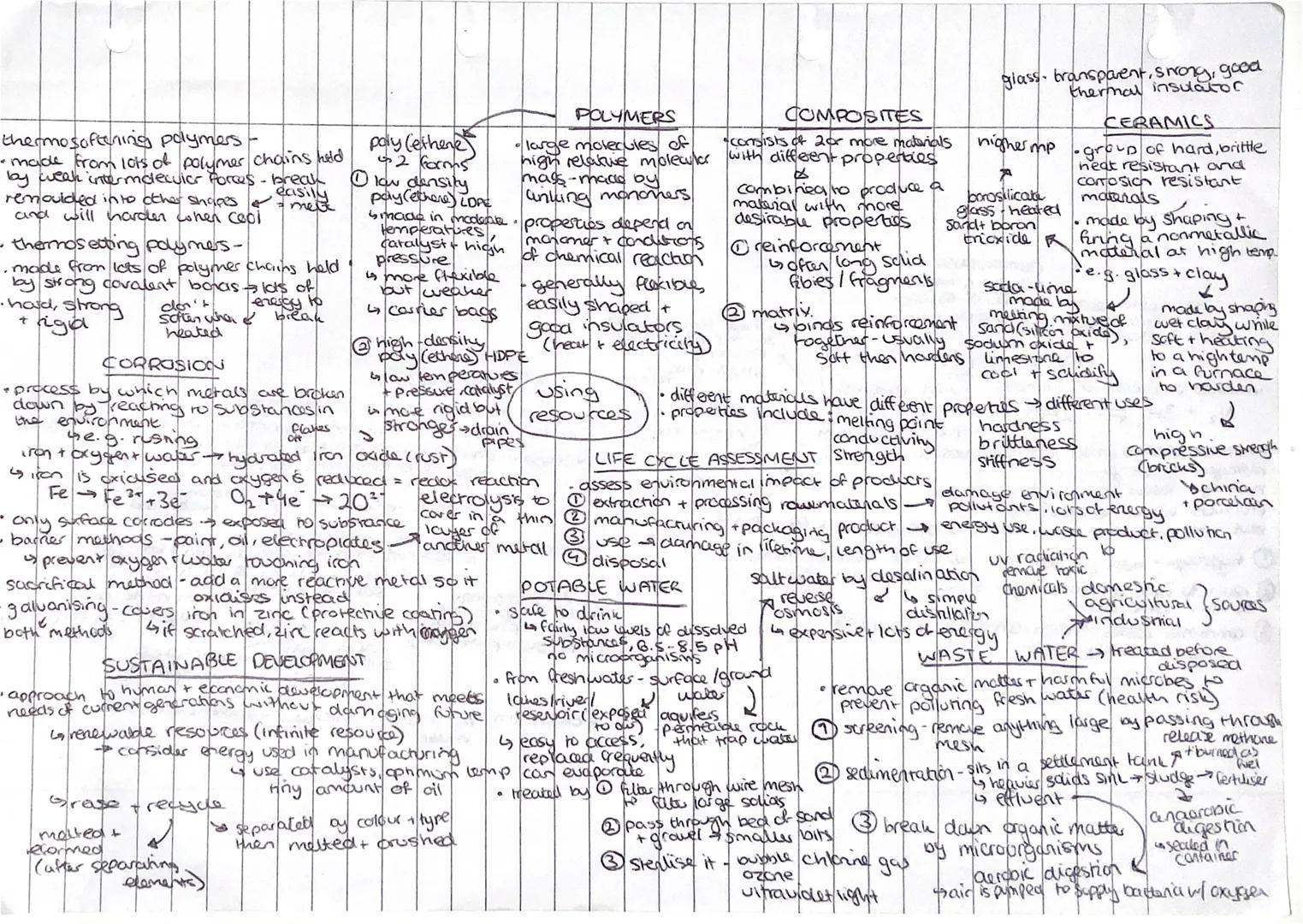
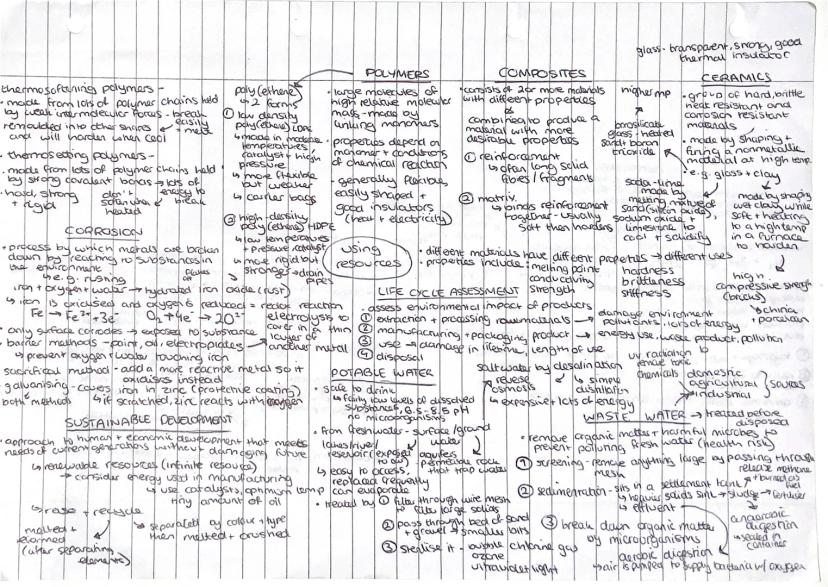
Different materials have properties that make them perfect for specific jobs! Thermosoftening polymers have weak intermolecular forces, so they melt and can be remoulded. Thermosetting polymers have strong covalent bonds and don't soften easily once set.
Corrosion breaks down metals through reaction with environmental substances. Rusting happens when iron reacts with oxygen and water. You can prevent this using barrier methods (paint, oil), sacrificial methods (more reactive metals), or galvanising (zinc coating).
Composites combine materials with different properties to create something with more desirable characteristics. They consist of reinforcement (strong fibres) held together by a softer matrix that hardens around them.
Potable water is safe to drink with low dissolved substances and pH between 6.5-8.5. Fresh water gets treated through filtration and sterilisation with chlorine or UV light. Wastewater treatment removes organic matter and harmful microbes through screening, sedimentation, and aerobic digestion.
Key Tip: Life cycle assessments consider environmental impact from raw material extraction right through to disposal - it's all about sustainable development!
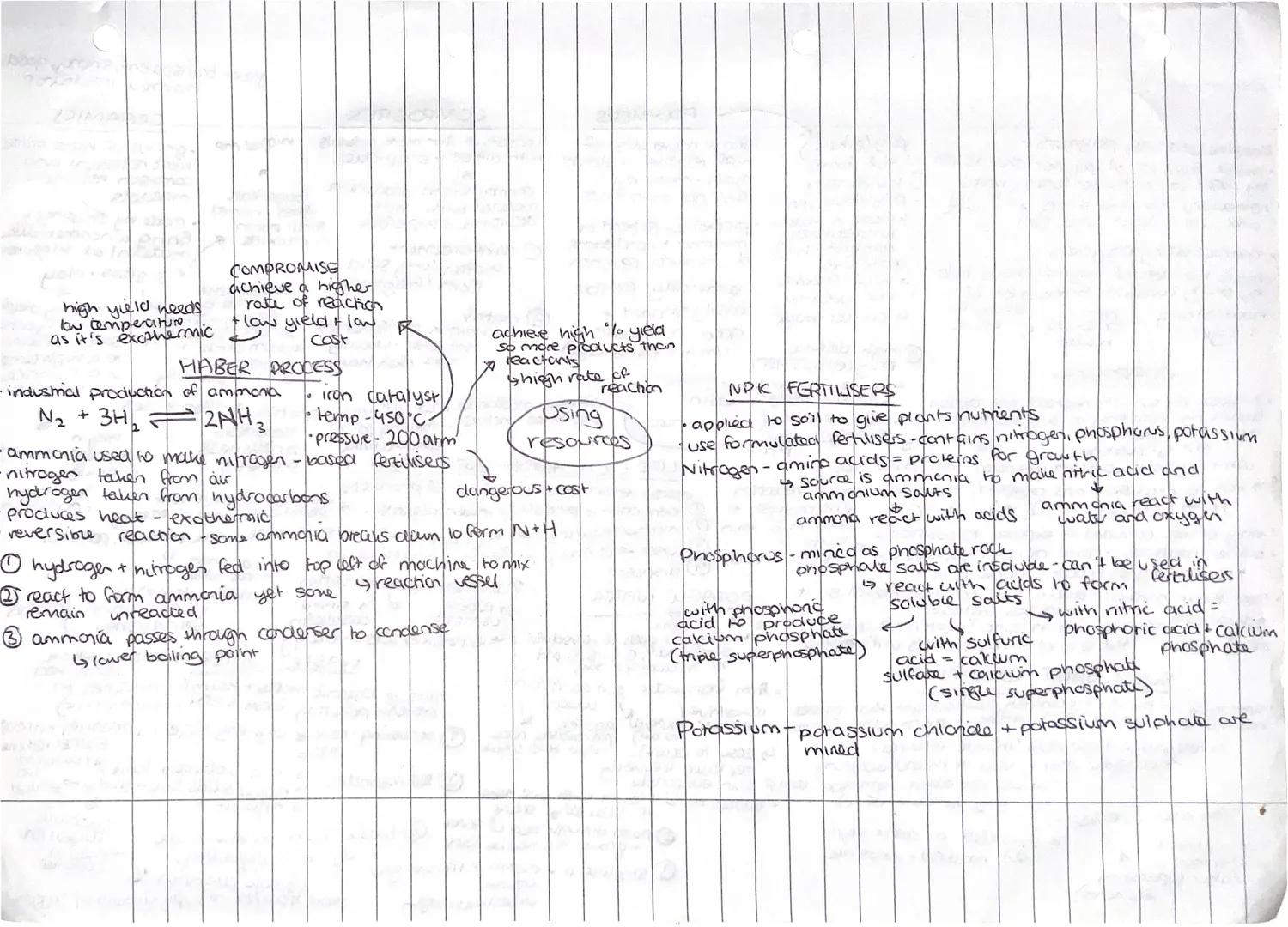
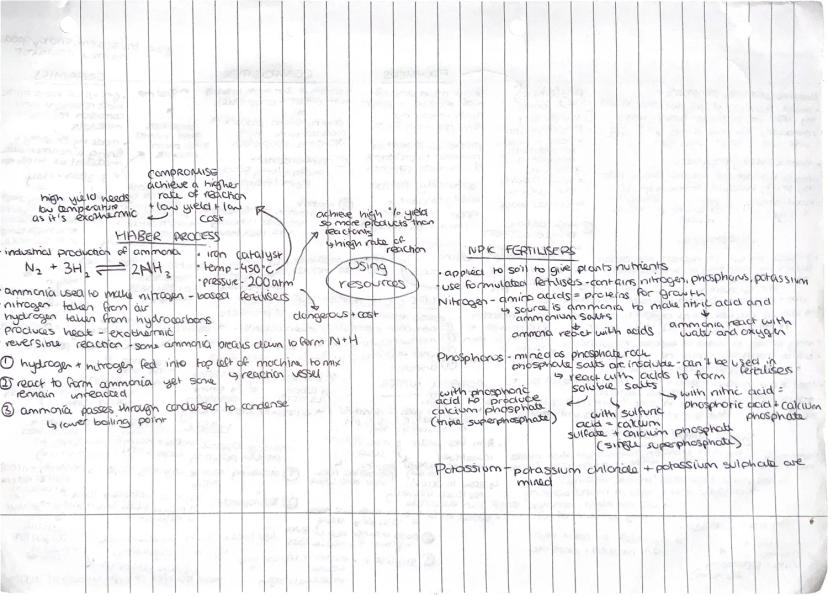
The Haber Process produces ammonia for fertilisers by combining nitrogen and hydrogen using an iron catalyst at 450°C and 200 atmospheres pressure. It's a brilliant example of industrial compromise - balancing yield, rate, and cost.
This reversible, exothermic reaction creates a dilemma: high yield needs low temperature, but high reaction rate needs high temperature. The chosen conditions give decent yield and rate without being too expensive or dangerous.
NPK fertilisers provide essential nutrients for plant growth. Nitrogen comes from ammonia converted to nitric acid and ammonium salts. Phosphorus gets extracted from phosphate rock and reacted with acids to make soluble salts. Potassium compounds like potassium chloride are simply mined.
The key is making insoluble compounds soluble so plants can actually absorb them. Phosphate rock is useless to plants until it's converted into water-soluble forms through reactions with sulfuric or phosphoric acid.
Key Tip: Industrial processes always involve compromises between ideal conditions and practical limitations like cost, safety, and equipment constraints!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
oliviaa2008
@oliviaa2008
Get ready to master the essential chemistry topics you'll need for your GCSEs! This comprehensive guide covers everything from organic chemistry and genetic engineering to industrial processes and chemical analysis - all the key concepts that'll help you ace your... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered how plastics are made or why ethanol burns so well? Organic chemistry is all about carbon-based compounds that form the building blocks of everything from fuels to medicines. You'll find these molecules everywhere in daily life.
Alcohols like ethanol have the functional group -OH and follow the formula C<sub>n</sub>H<sub>2n+1</sub>OH. They're flammable, soluble in water, and can be oxidised to form carboxylic acids. Ethanol is particularly important - it's used in alcoholic drinks, as biofuel, and as a chemical feedstock for making other compounds.
Alkanes are saturated hydrocarbons that get more viscous and less flammable as their chain length increases. Through cracking (thermal decomposition), we can break long alkanes into shorter, more useful alkanes and alkenes. This process uses either steam cracking or catalytic cracking with aluminium oxide.
Addition polymers form when alkene monomers join together after their double bonds split. Meanwhile, condensation polymers like polyesters form when monomers with at least two functional groups join, producing water as a byproduct.
Key Tip: Remember that alkenes are more reactive than alkanes because of their double bonds - they'll turn bromine water from orange to colourless!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Your DNA holds the instruction manual for making you who you are! Genetic engineering allows scientists to cut genes from one organism and transfer them to another, creating everything from insulin for diabetics to disease-resistant crops.
DNA is a polymer made of nucleotides, each containing a phosphate, sugar, and base. The genetic code uses sequences of three bases (codons) to specify which amino acid gets added during protein synthesis. This happens through transcription (copying DNA to mRNA) and translation (using mRNA to build proteins).
Variation within populations comes from genes and environment interacting. Natural selection means better-adapted organisms survive and reproduce, passing on favourable traits. This leads to evolution - a process proven by fossil records and observable in antibiotic resistance.
Meiosis produces four genetically unique gametes through two divisions, whilst sexual reproduction creates genetically different offspring. Asexual reproduction is quicker but produces identical clones with no variation.
Key Tip: Mutations in DNA can change amino acid sequences, potentially altering protein function - but most mutations actually have no effect!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Want to control chemical reactions like a pro? Le Chatelier's Principle is your secret weapon - it predicts how equilibrium shifts when you change temperature, pressure, or concentration.
Reversible reactions reach equilibrium when forward and backward reaction rates become equal. Initially, you have loads of reactants and a fast forward reaction. As products form, the forward reaction slows whilst the backward reaction speeds up until they balance out.
Temperature changes shift equilibrium towards the reaction that counteracts the change. If you increase temperature, equilibrium moves towards the endothermic direction to absorb the extra heat. Pressure increases favour the side with fewer gas molecules.
Collision theory explains reaction rates - particles must collide with sufficient activation energy to react. You can speed up reactions by increasing concentration, pressure, surface area, temperature, or adding a catalyst. Catalysts provide alternative reaction pathways with lower activation energy.
Key Tip: At equilibrium, concentrations stay constant but aren't necessarily equal - there could be more products than reactants or vice versa!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Testing for different ions and substances is like being a chemical detective! Flame tests give specific colours for metal ions - lithium burns crimson, calcium gives orange-red, whilst sodium produces a bright yellow flame.
For anion tests, add dilute HCl to carbonates and pass the gas through limewater (turns cloudy). Sulfate ions form white precipitates with barium chloride, whilst halide ions create different coloured precipitates with silver nitrate - chloride gives white, bromide gives cream, and iodide gives yellow.
Flame emission spectroscopy is more accurate than simple flame tests because it detects specific wavelengths of light emitted by heated metal ions. Each metal produces a unique spectrum pattern, and light intensity indicates concentration.
Chromatography separates mixtures by dissolving substances in a solvent. More soluble substances travel further up the paper. Pure substances melt and boil at specific temperatures, whilst impure substances melt and boil over temperature ranges.
Key Tip: Always use pencil for chromatography baselines because ink would interfere with your results by dissolving in the solvent!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Different materials have properties that make them perfect for specific jobs! Thermosoftening polymers have weak intermolecular forces, so they melt and can be remoulded. Thermosetting polymers have strong covalent bonds and don't soften easily once set.
Corrosion breaks down metals through reaction with environmental substances. Rusting happens when iron reacts with oxygen and water. You can prevent this using barrier methods (paint, oil), sacrificial methods (more reactive metals), or galvanising (zinc coating).
Composites combine materials with different properties to create something with more desirable characteristics. They consist of reinforcement (strong fibres) held together by a softer matrix that hardens around them.
Potable water is safe to drink with low dissolved substances and pH between 6.5-8.5. Fresh water gets treated through filtration and sterilisation with chlorine or UV light. Wastewater treatment removes organic matter and harmful microbes through screening, sedimentation, and aerobic digestion.
Key Tip: Life cycle assessments consider environmental impact from raw material extraction right through to disposal - it's all about sustainable development!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The Haber Process produces ammonia for fertilisers by combining nitrogen and hydrogen using an iron catalyst at 450°C and 200 atmospheres pressure. It's a brilliant example of industrial compromise - balancing yield, rate, and cost.
This reversible, exothermic reaction creates a dilemma: high yield needs low temperature, but high reaction rate needs high temperature. The chosen conditions give decent yield and rate without being too expensive or dangerous.
NPK fertilisers provide essential nutrients for plant growth. Nitrogen comes from ammonia converted to nitric acid and ammonium salts. Phosphorus gets extracted from phosphate rock and reacted with acids to make soluble salts. Potassium compounds like potassium chloride are simply mined.
The key is making insoluble compounds soluble so plants can actually absorb them. Phosphate rock is useless to plants until it's converted into water-soluble forms through reactions with sulfuric or phosphoric acid.
Key Tip: Industrial processes always involve compromises between ideal conditions and practical limitations like cost, safety, and equipment constraints!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
3
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user