Enzymes are absolutely essential for life - they're the protein... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
73
•
6 Feb 2026
•
Gabriela
@gabriela.my.school.journey16
Enzymes are absolutely essential for life - they're the protein... Show more








Think of enzymes as the ultimate efficiency experts of the biological world. These biological catalysts are large globular proteins that dramatically speed up chemical reactions by lowering the activation energy needed to get reactions started.
Here's the clever bit: enzymes don't get used up in the process. They emerge unchanged and ready to catalyse the same reaction again and again. Most of each enzyme is just there to maintain its precise shape - only a tiny region called the active site actually does the catalytic work.
The energy diagram shows this beautifully - with an enzyme present, reactions need much less energy to get going. This means reactions that would normally crawl along at a snail's pace can happen rapidly enough to sustain life.
Key Insight: Enzymes are soluble in water due to hydrophilic side groups, making them perfect for working in aqueous cellular environments.
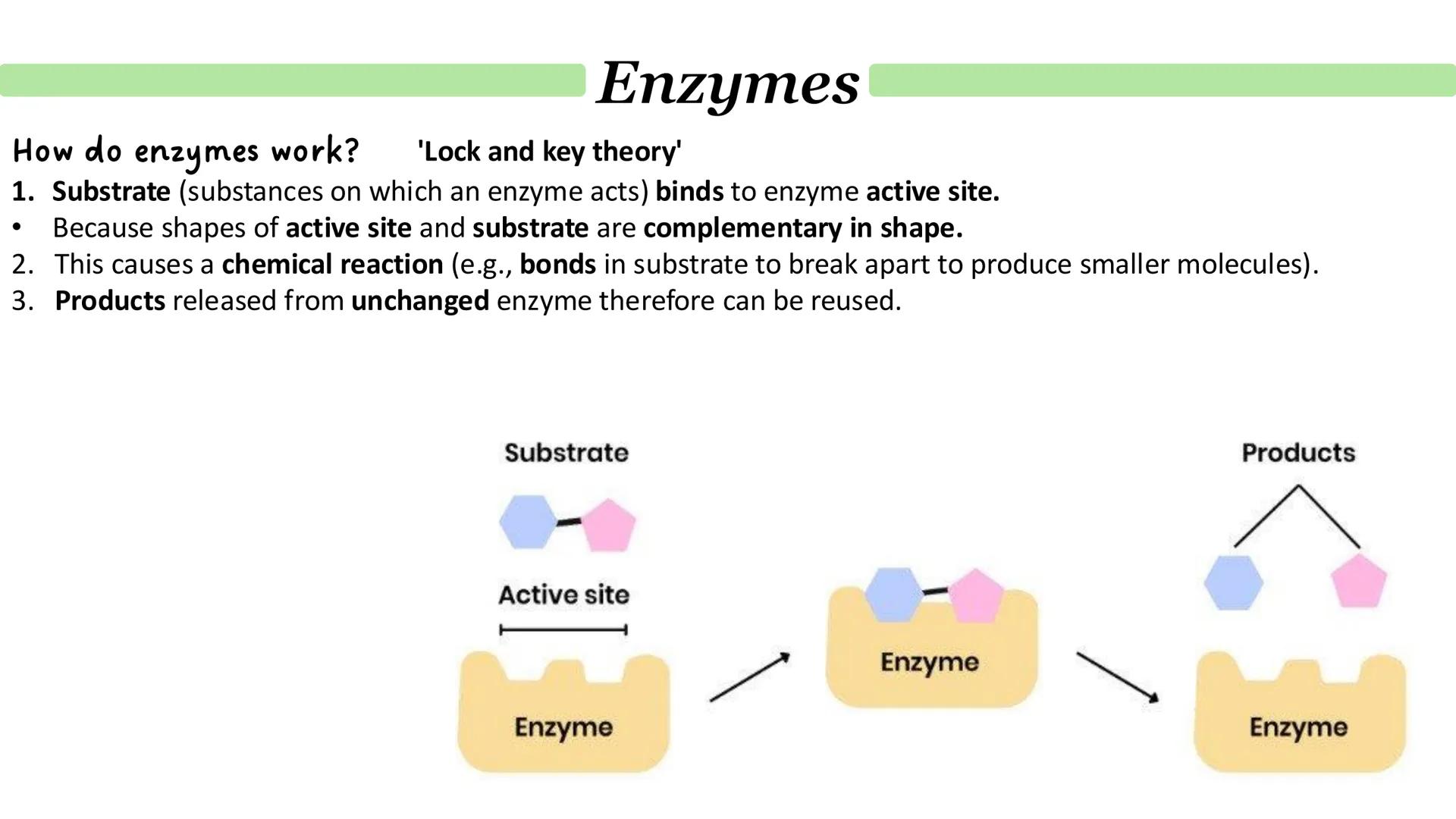
The lock and key theory was biology's first attempt to explain enzyme specificity. It's beautifully simple: the enzyme's active site acts like a lock, and only one specific substrate (the key) fits perfectly.
The process follows three straightforward steps. First, the substrate binds to the complementary active site. Then, the binding triggers a chemical reaction that breaks or forms bonds. Finally, the products are released, leaving the enzyme unchanged and ready for another round.
Whilst this model helps visualise enzyme action, it's now considered outdated. Real enzymes are far more flexible and dynamic than rigid locks.
Remember: This theory explains enzyme specificity well but fails to account for the flexibility we now know enzymes possess.
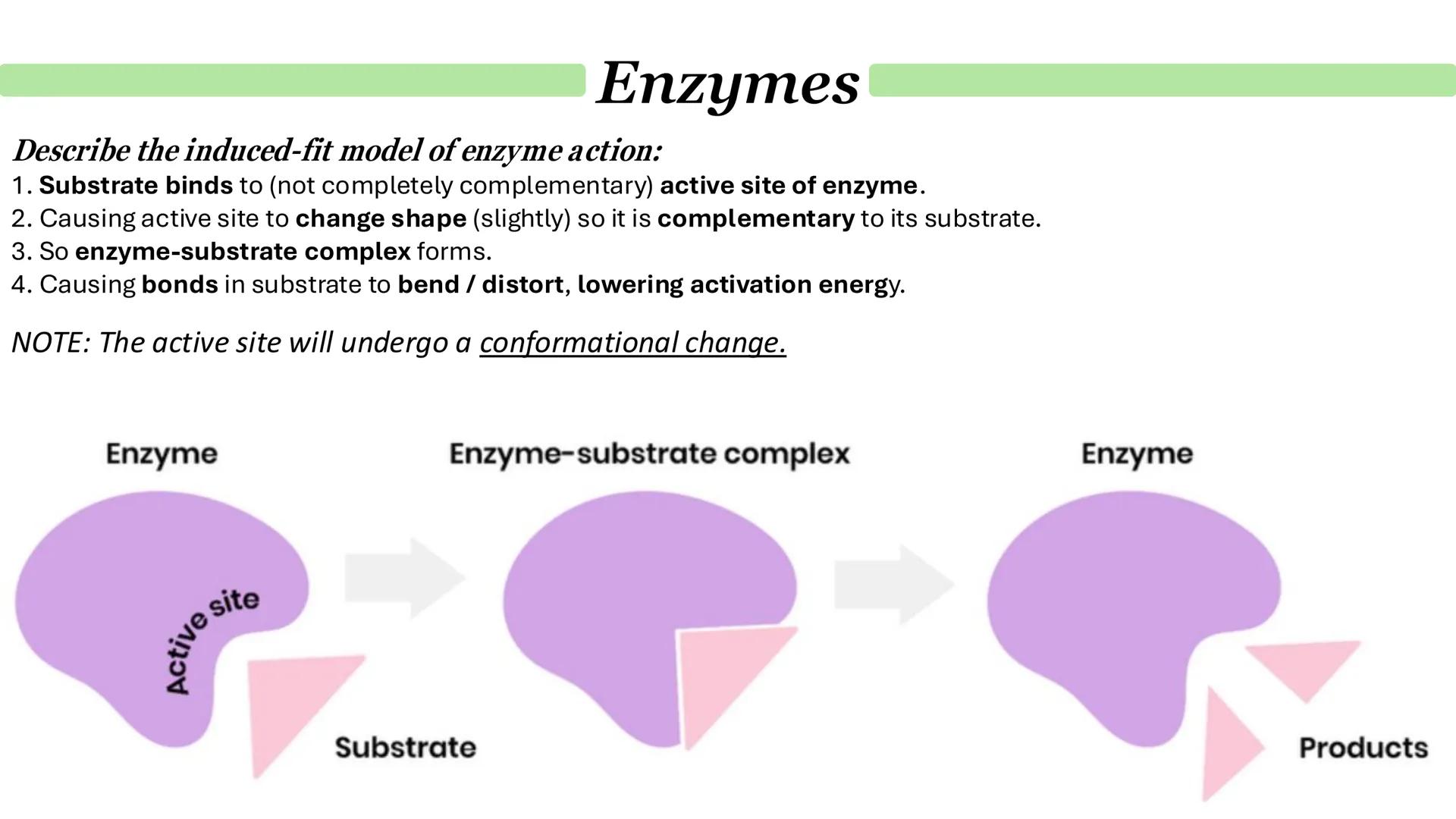
The induced-fit model revolutionised our understanding of enzyme action. Unlike the rigid lock and key model, this theory recognises that enzymes are flexible molecules that change shape when substrates approach.
Here's how it works: the substrate initially binds to an active site that's not perfectly complementary. This binding causes the enzyme-substrate complex to form as the active site moulds itself around the substrate like a glove fitting a hand.
This shape change is crucial because it distorts bonds in the substrate, making them easier to break and lowering the activation energy. The conformational change in the enzyme is what makes catalysis so efficient.
Exam Tip: The induced-fit model explains both enzyme specificity and how catalysis actually occurs - perfect for those tricky mechanism questions.

Scientific models evolve as we gather more evidence, and enzyme theory is a perfect example. The lock and key model seemed logical initially but couldn't explain several key observations about enzyme behaviour.
The induced-fit model solves these problems brilliantly. It explains why some enzymes show broad specificity - lipase, for instance, can work on various lipids because the active site can adjust to accommodate slightly different substrates.
More importantly, it explains the actual mechanism of catalysis. The conformational changes stress the substrate's bonds, increasing reactivity and making the reaction more likely to occur.
Evidence supporting this model includes observations that molecules binding elsewhere on the enzyme can affect activity - something impossible if enzymes were rigid structures.
Think About It: When other molecules affect enzyme activity by binding away from the active site, it proves enzymes must be flexible, shape-changing molecules.

Enzyme specificity is all about molecular architecture. Each enzyme's tertiary structure creates a unique active site shape that's complementary to one specific substrate - it's like having a molecular signature.
This specificity stems from the primary structure - the exact sequence of amino acids. Change just one amino acid in the active site, and you might completely destroy the enzyme's function.
The consequences of amino acid changes are severe. The altered amino acid might no longer bind properly to the substrate, or it could disrupt hydrogen bonding patterns that maintain the enzyme's shape. Either way, the result is often a non-functional enzyme.
Real-World Connection: Many genetic diseases result from single amino acid changes in important enzymes - highlighting just how precise these molecular machines need to be.
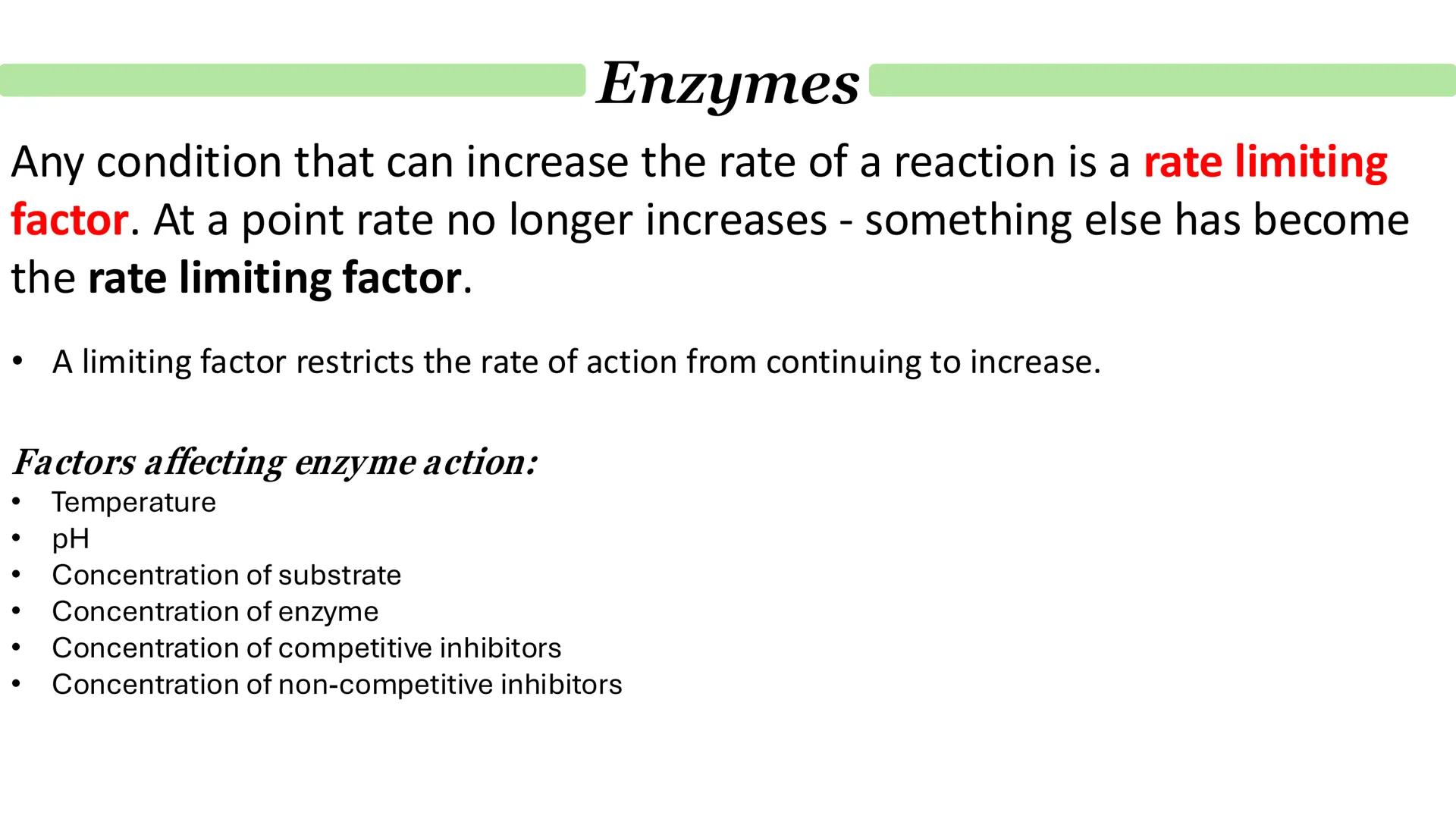
Understanding what affects enzyme activity is crucial because cells need to control reaction rates precisely. Several factors can become rate-limiting - meaning they restrict how fast the reaction can proceed.
The main factors affecting enzyme action include temperature, pH, and the concentrations of enzyme, substrate, and various inhibitors. Each of these can either speed up or slow down enzymatic reactions.
When conditions are optimised, the reaction rate increases until something else becomes the limiting factor. This creates the characteristic curves you'll see in enzyme kinetics graphs.
Exam Strategy: Learn to identify which factor is limiting the reaction rate from graph shapes - it's a common exam question format.
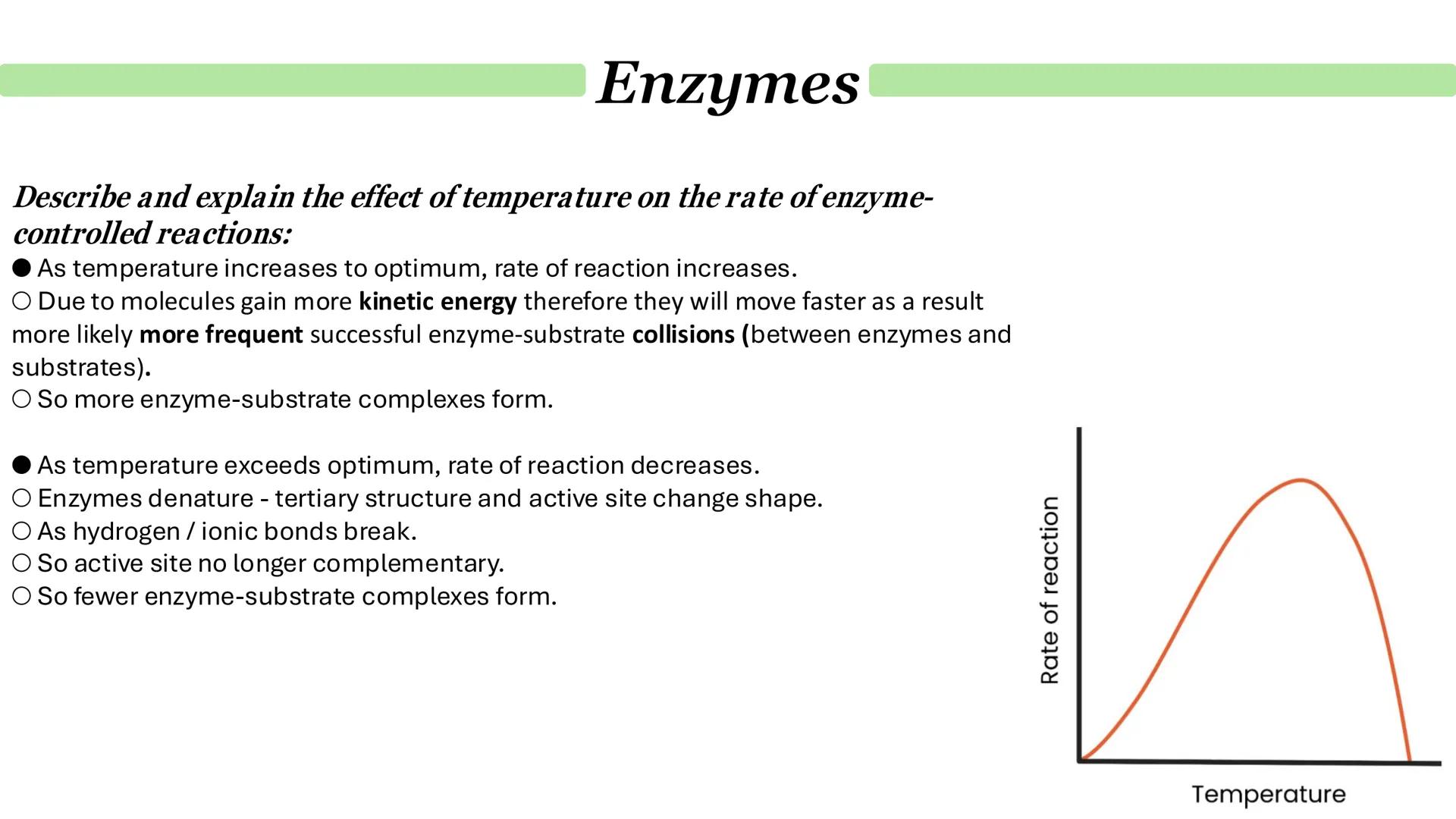
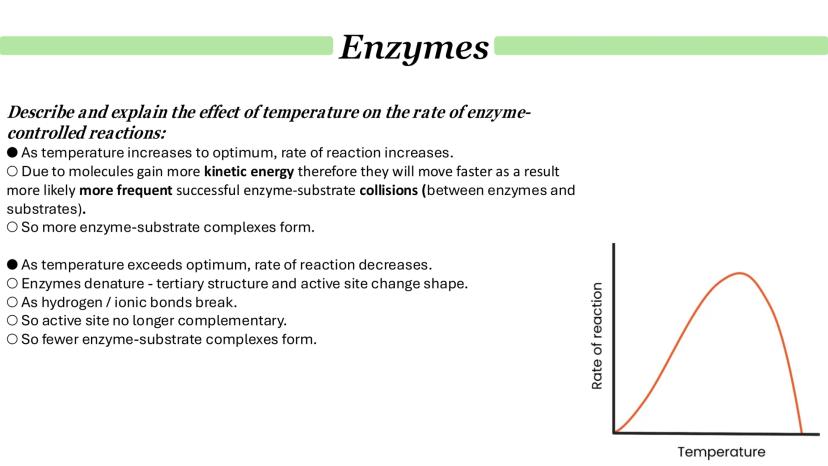
Temperature creates a fascinating balancing act in enzyme kinetics. As temperature increases towards the optimum, reaction rates soar because molecules gain kinetic energy and collide more frequently, forming more enzyme-substrate complexes.
However, push the temperature beyond the optimum and disaster strikes. Enzyme denaturation begins as hydrogen and ionic bonds break, causing the tertiary structure to unfold. The active site loses its complementary shape, and fewer enzyme-substrate complexes can form.
This creates the classic bell-shaped curve you'll see in temperature-activity graphs. The ascending portion shows increased molecular motion, whilst the descending portion shows progressive denaturation.
Remember: Denaturation is usually irreversible - once an enzyme unfolds due to excessive heat, it won't regain its activity when cooled.

pH changes can make or break enzyme function. Small deviations from the optimum pH might only slow the reaction temporarily, but extreme pH changes cause irreversible denaturation.
The mechanism is similar to temperature effects. As pH moves away from the optimum, hydrogen and ionic bonds that maintain the enzyme's shape begin to break. The tertiary structure destabilises, the active site changes shape, and fewer enzyme-substrate complexes can form.
Each enzyme has evolved to work best at a specific pH that matches its cellular environment. Pepsin loves the acidic stomach environment, whilst other enzymes prefer neutral or slightly alkaline conditions.
Key Point: Unlike temperature denaturation, extreme pH denaturation is also irreversible - the enzyme won't refold properly even if pH is restored.
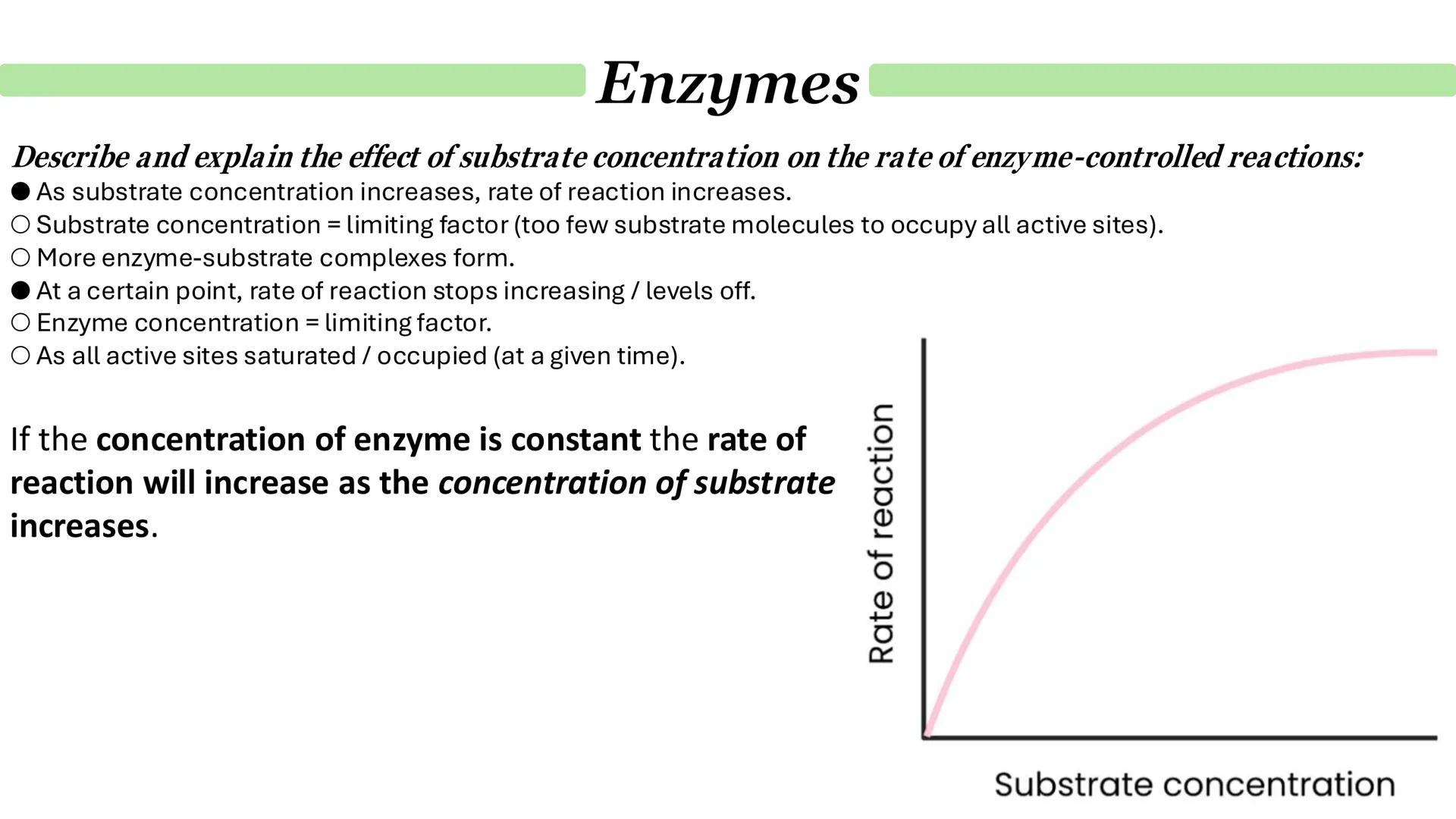
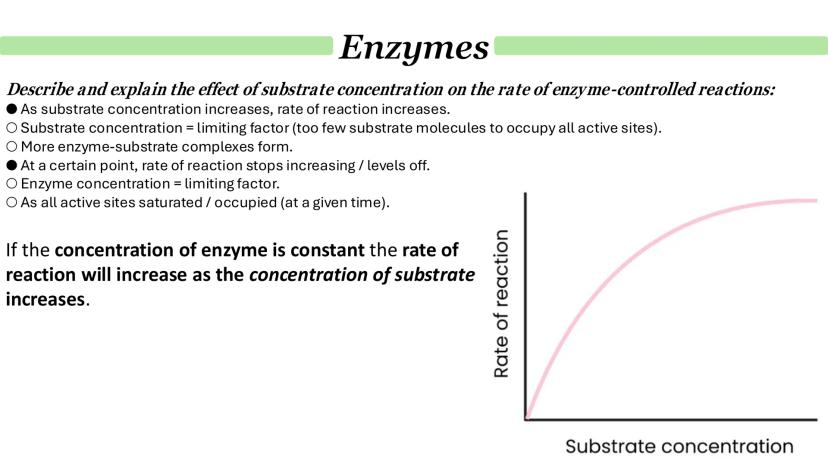
Substrate concentration effects follow a predictable pattern that's perfect for understanding limiting factors. Initially, as substrate concentration increases, reaction rates climb steadily because more substrate molecules are available to form enzyme-substrate complexes.
However, this can't continue indefinitely. Eventually, the curve levels off as enzyme concentration becomes the limiting factor. At this point, all active sites are saturated - they're working flat out and can't process substrates any faster.
This saturation point is crucial for understanding enzyme kinetics. No matter how much more substrate you add, the reaction rate won't increase further until you add more enzyme.
Graph Reading Tip: The point where the curve levels off shows you've reached enzyme saturation - a key concept for interpreting kinetics data.
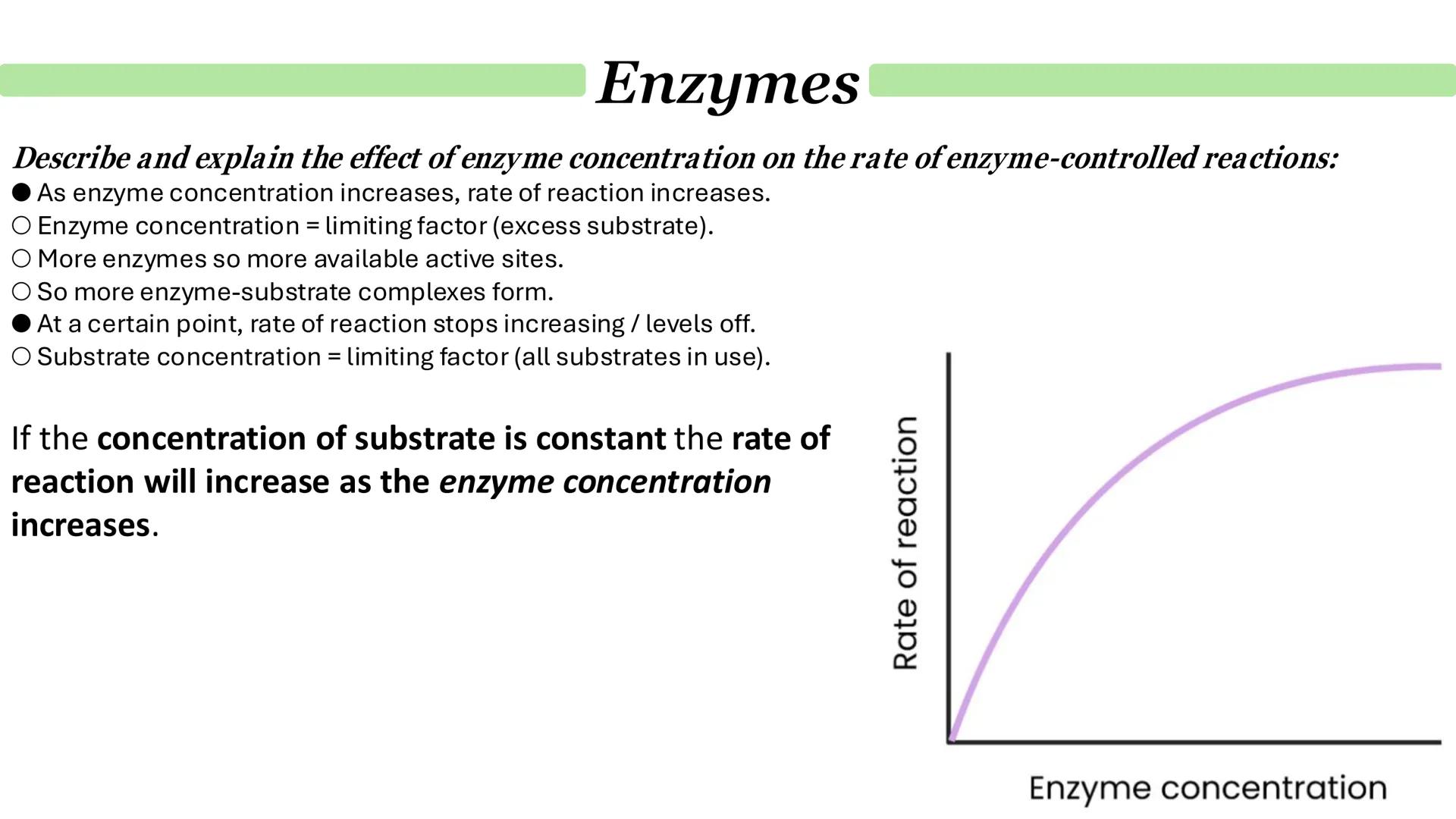
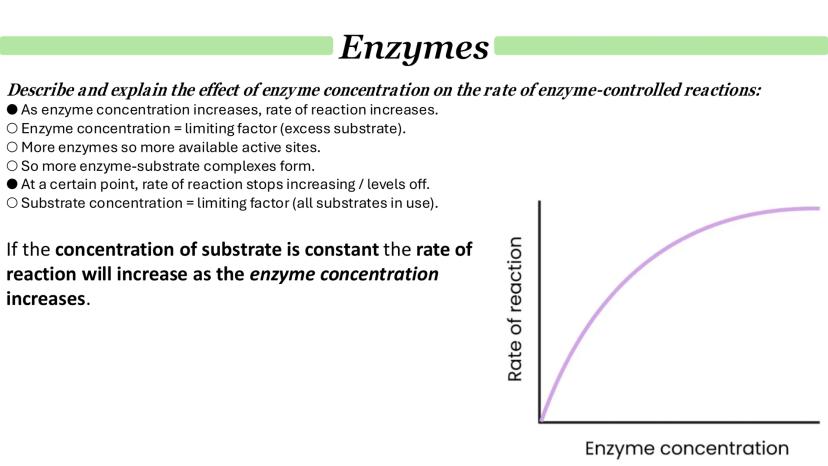
Enzyme concentration effects mirror substrate concentration patterns but in reverse. When substrate is abundant but enzyme is scarce, adding more enzyme directly increases the reaction rate by providing more active sites.
The relationship is linear initially - double the enzyme concentration, double the reaction rate. This happens because there's excess substrate available, so enzyme concentration is the limiting factor.
Eventually, though, the curve levels off again. This time it's because substrate concentration becomes limiting - all the substrate molecules are already being processed as fast as they're available.
Understanding these concentration effects helps explain how cells regulate metabolism by controlling enzyme levels.
Clinical Connection: Many metabolic disorders involve enzyme deficiencies where insufficient enzyme concentration limits essential biochemical pathways.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Gabriela
@gabriela.my.school.journey16
Enzymes are absolutely essential for life - they're the protein catalysts that speed up virtually every chemical reaction in your body. Understanding how they work and what affects their activity is crucial for A-level Biology, as these concepts appear in... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of enzymes as the ultimate efficiency experts of the biological world. These biological catalysts are large globular proteins that dramatically speed up chemical reactions by lowering the activation energy needed to get reactions started.
Here's the clever bit: enzymes don't get used up in the process. They emerge unchanged and ready to catalyse the same reaction again and again. Most of each enzyme is just there to maintain its precise shape - only a tiny region called the active site actually does the catalytic work.
The energy diagram shows this beautifully - with an enzyme present, reactions need much less energy to get going. This means reactions that would normally crawl along at a snail's pace can happen rapidly enough to sustain life.
Key Insight: Enzymes are soluble in water due to hydrophilic side groups, making them perfect for working in aqueous cellular environments.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The lock and key theory was biology's first attempt to explain enzyme specificity. It's beautifully simple: the enzyme's active site acts like a lock, and only one specific substrate (the key) fits perfectly.
The process follows three straightforward steps. First, the substrate binds to the complementary active site. Then, the binding triggers a chemical reaction that breaks or forms bonds. Finally, the products are released, leaving the enzyme unchanged and ready for another round.
Whilst this model helps visualise enzyme action, it's now considered outdated. Real enzymes are far more flexible and dynamic than rigid locks.
Remember: This theory explains enzyme specificity well but fails to account for the flexibility we now know enzymes possess.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The induced-fit model revolutionised our understanding of enzyme action. Unlike the rigid lock and key model, this theory recognises that enzymes are flexible molecules that change shape when substrates approach.
Here's how it works: the substrate initially binds to an active site that's not perfectly complementary. This binding causes the enzyme-substrate complex to form as the active site moulds itself around the substrate like a glove fitting a hand.
This shape change is crucial because it distorts bonds in the substrate, making them easier to break and lowering the activation energy. The conformational change in the enzyme is what makes catalysis so efficient.
Exam Tip: The induced-fit model explains both enzyme specificity and how catalysis actually occurs - perfect for those tricky mechanism questions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Scientific models evolve as we gather more evidence, and enzyme theory is a perfect example. The lock and key model seemed logical initially but couldn't explain several key observations about enzyme behaviour.
The induced-fit model solves these problems brilliantly. It explains why some enzymes show broad specificity - lipase, for instance, can work on various lipids because the active site can adjust to accommodate slightly different substrates.
More importantly, it explains the actual mechanism of catalysis. The conformational changes stress the substrate's bonds, increasing reactivity and making the reaction more likely to occur.
Evidence supporting this model includes observations that molecules binding elsewhere on the enzyme can affect activity - something impossible if enzymes were rigid structures.
Think About It: When other molecules affect enzyme activity by binding away from the active site, it proves enzymes must be flexible, shape-changing molecules.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Enzyme specificity is all about molecular architecture. Each enzyme's tertiary structure creates a unique active site shape that's complementary to one specific substrate - it's like having a molecular signature.
This specificity stems from the primary structure - the exact sequence of amino acids. Change just one amino acid in the active site, and you might completely destroy the enzyme's function.
The consequences of amino acid changes are severe. The altered amino acid might no longer bind properly to the substrate, or it could disrupt hydrogen bonding patterns that maintain the enzyme's shape. Either way, the result is often a non-functional enzyme.
Real-World Connection: Many genetic diseases result from single amino acid changes in important enzymes - highlighting just how precise these molecular machines need to be.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Understanding what affects enzyme activity is crucial because cells need to control reaction rates precisely. Several factors can become rate-limiting - meaning they restrict how fast the reaction can proceed.
The main factors affecting enzyme action include temperature, pH, and the concentrations of enzyme, substrate, and various inhibitors. Each of these can either speed up or slow down enzymatic reactions.
When conditions are optimised, the reaction rate increases until something else becomes the limiting factor. This creates the characteristic curves you'll see in enzyme kinetics graphs.
Exam Strategy: Learn to identify which factor is limiting the reaction rate from graph shapes - it's a common exam question format.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Temperature creates a fascinating balancing act in enzyme kinetics. As temperature increases towards the optimum, reaction rates soar because molecules gain kinetic energy and collide more frequently, forming more enzyme-substrate complexes.
However, push the temperature beyond the optimum and disaster strikes. Enzyme denaturation begins as hydrogen and ionic bonds break, causing the tertiary structure to unfold. The active site loses its complementary shape, and fewer enzyme-substrate complexes can form.
This creates the classic bell-shaped curve you'll see in temperature-activity graphs. The ascending portion shows increased molecular motion, whilst the descending portion shows progressive denaturation.
Remember: Denaturation is usually irreversible - once an enzyme unfolds due to excessive heat, it won't regain its activity when cooled.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
pH changes can make or break enzyme function. Small deviations from the optimum pH might only slow the reaction temporarily, but extreme pH changes cause irreversible denaturation.
The mechanism is similar to temperature effects. As pH moves away from the optimum, hydrogen and ionic bonds that maintain the enzyme's shape begin to break. The tertiary structure destabilises, the active site changes shape, and fewer enzyme-substrate complexes can form.
Each enzyme has evolved to work best at a specific pH that matches its cellular environment. Pepsin loves the acidic stomach environment, whilst other enzymes prefer neutral or slightly alkaline conditions.
Key Point: Unlike temperature denaturation, extreme pH denaturation is also irreversible - the enzyme won't refold properly even if pH is restored.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Substrate concentration effects follow a predictable pattern that's perfect for understanding limiting factors. Initially, as substrate concentration increases, reaction rates climb steadily because more substrate molecules are available to form enzyme-substrate complexes.
However, this can't continue indefinitely. Eventually, the curve levels off as enzyme concentration becomes the limiting factor. At this point, all active sites are saturated - they're working flat out and can't process substrates any faster.
This saturation point is crucial for understanding enzyme kinetics. No matter how much more substrate you add, the reaction rate won't increase further until you add more enzyme.
Graph Reading Tip: The point where the curve levels off shows you've reached enzyme saturation - a key concept for interpreting kinetics data.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Enzyme concentration effects mirror substrate concentration patterns but in reverse. When substrate is abundant but enzyme is scarce, adding more enzyme directly increases the reaction rate by providing more active sites.
The relationship is linear initially - double the enzyme concentration, double the reaction rate. This happens because there's excess substrate available, so enzyme concentration is the limiting factor.
Eventually, though, the curve levels off again. This time it's because substrate concentration becomes limiting - all the substrate molecules are already being processed as fast as they're available.
Understanding these concentration effects helps explain how cells regulate metabolism by controlling enzyme levels.
Clinical Connection: Many metabolic disorders involve enzyme deficiencies where insufficient enzyme concentration limits essential biochemical pathways.
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
0
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user