Ever wondered what makes up all living things at the... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
Classic Dramatic Literature
Modern Lyric Poetry
Influential English-Language Authors
Classic and Contemporary Novels
Literary Character Analysis
Romantic and Love Poetry
Reading Analysis and Interpretation
Evidence Analysis and Integration
Author's Stylistic Elements
Figurative Language and Rhetoric
Show all topics
Human Organ Systems
Cellular Organization and Development
Biomolecular Structure and Organization
Enzyme Structure and Regulation
Cellular Organization Types
Biological Homeostatic Processes
Cellular Membrane Structure
Autotrophic Energy Processes
Environmental Sustainability and Impact
Neural Communication Systems
Show all topics
Social Sciences Research & Practice
Social Structure and Mobility
Classic Social Influence Experiments
Social Systems Theories
Family and Relationship Dynamics
Memory Systems and Processes
Neural Bases of Behavior
Social Influence and Attraction
Psychotherapeutic Approaches
Human Agency and Responsibility
Show all topics
Chemical Sciences and Applications
Chemical Bond Types and Properties
Organic Functional Groups
Atomic Structure and Composition
Chromatographic Separation Principles
Chemical Compound Classifications
Electrochemical Cell Systems
Periodic Table Organization
Chemical Reaction Kinetics
Chemical Equation Conservation
Show all topics
Nazi Germany and Holocaust 1933-1945
World Wars and Peace Treaties
European Monarchs and Statesmen
Cold War Global Tensions
Medieval Institutions and Systems
European Renaissance and Enlightenment
Modern Global Environmental-Health Challenges
Modern Military Conflicts
Medieval Migration and Invasions
World Wars Era and Impact
Show all topics
106
•
2 Feb 2026
•
Jasmin Thompson
@jasmintho_n26iu
Ever wondered what makes up all living things at the... Show more





All living organisms are remarkably similar when you look closely at their molecular structure. Whether you're examining a human, a houseplant, or bacteria, they're all built from the same types of biological molecules.
These molecules fall into two main categories: small molecules called monomers and massive molecules called polymers. Think of monomers as individual LEGO bricks, whilst polymers are the complete structures you build from joining loads of bricks together.
The most important groups you need to know are carbohydrates, lipids, and proteins. Each group has specific jobs - carbs provide energy, lipids store energy and form cell membranes, and proteins do almost everything else from building muscle to speeding up reactions.
💡 Key Point: Carbon atoms are special because they can form four bonds, allowing them to create the complex molecular frameworks that make life possible.
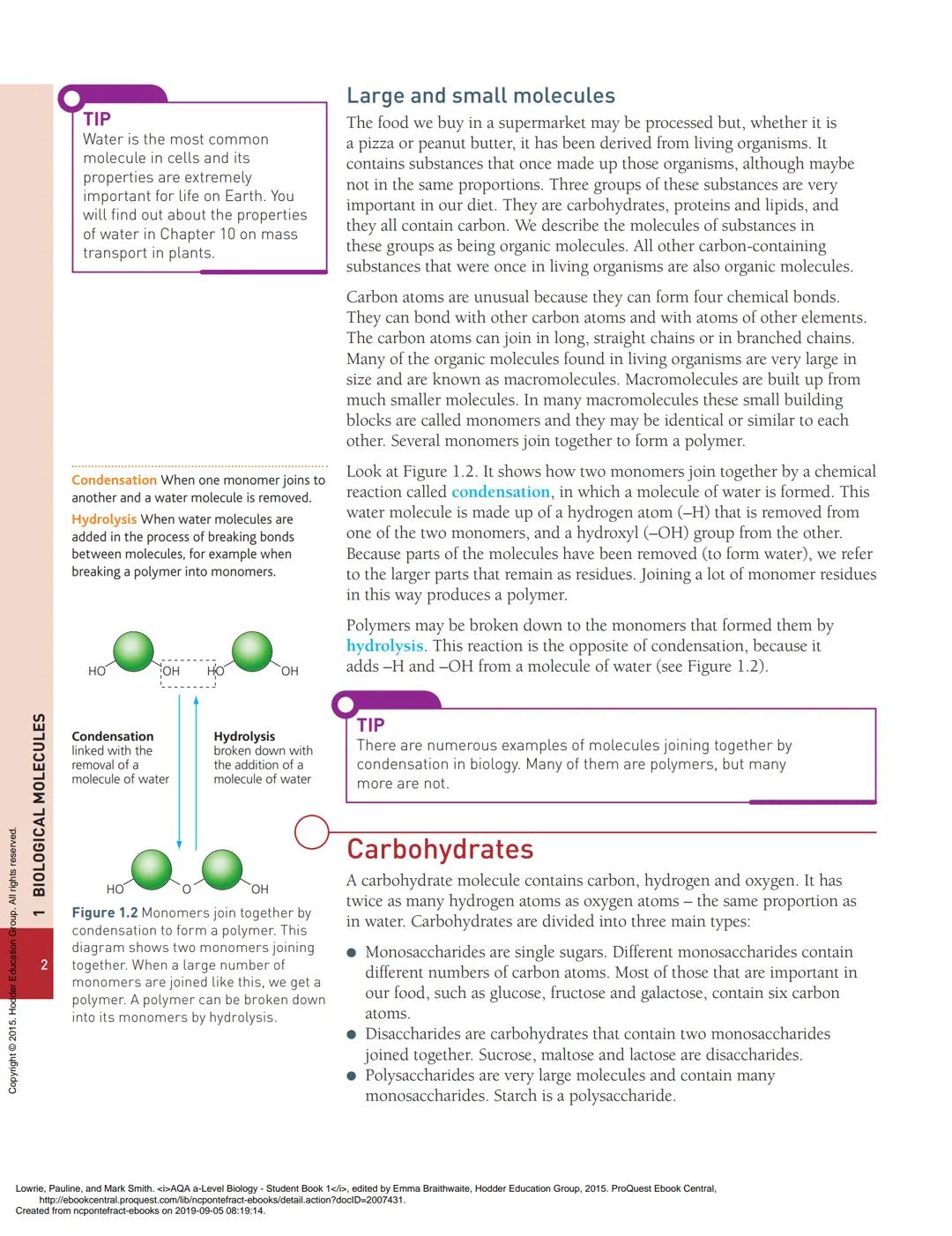
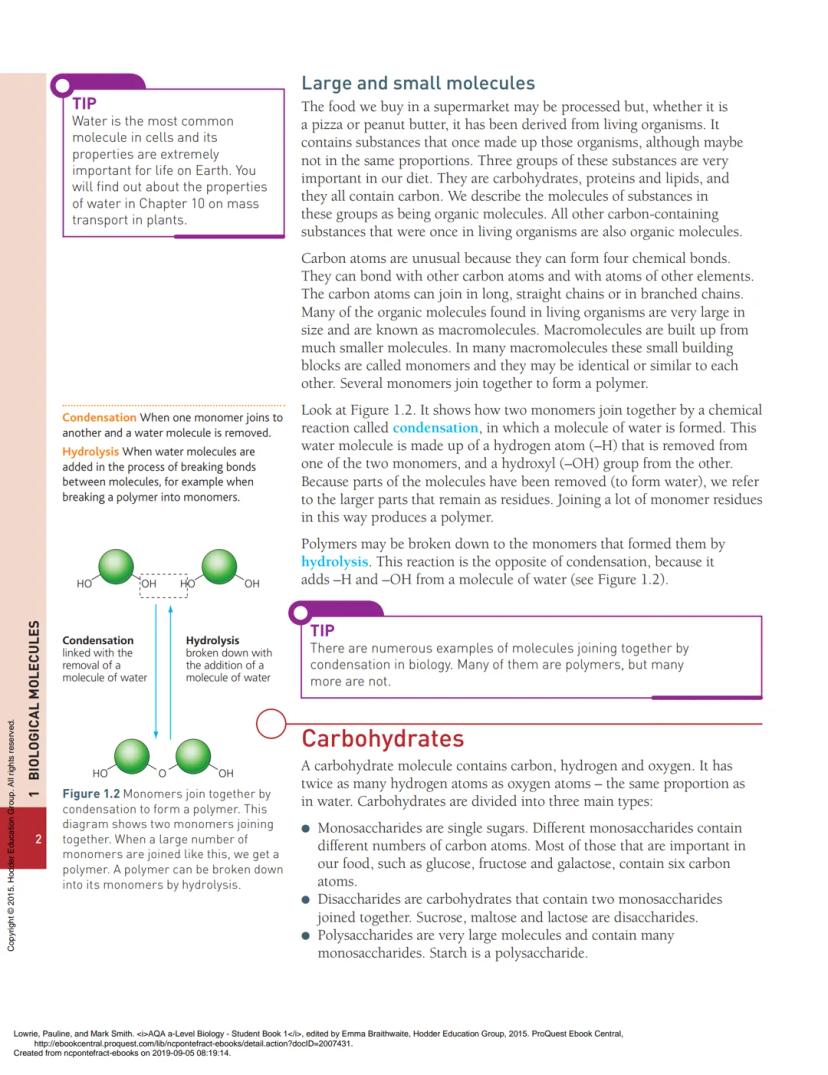
Macromolecules are built through a process called condensation, where two smaller molecules join together and kick out a water molecule in the process. It's like molecular matchmaking - one molecule loses a hydrogen (H), the other loses a hydroxyl group (OH), and together they form water (H₂O).
The opposite process is hydrolysis - breaking big molecules back down by adding water. Your digestive system uses hydrolysis constantly to break down food into smaller, usable pieces.
Carbohydrates contain carbon, hydrogen, and oxygen in a 2:1 ratio (twice as many hydrogens as oxygens). They come in three sizes: monosaccharides (single sugars like glucose), disaccharides (two sugars joined together like sucrose), and polysaccharides (massive chains like starch).
💡 Remember: Condensation reactions build molecules UP by removing water, whilst hydrolysis breaks molecules DOWN by adding water.

Glucose has the formula C₆H₁₂O₆, but there are actually two different versions: α-glucose and β-glucose. These are called isomers - same formula, different arrangement of atoms. The difference is tiny but crucial for how they behave.
When two glucose molecules join, they form a glycosidic bond through condensation. Two α-glucose molecules create maltose, whilst glucose can also join with other sugars like fructose to make sucrose (table sugar).
Reducing sugars like glucose, fructose, and maltose will turn Benedict's solution orange when heated - this happens because they can donate electrons (get reduced). Sucrose won't do this directly, but if you break it down with acid first, then it will test positive.
Different sugars have identical formulas but completely different properties because of how their atoms are arranged. It's like having the same ingredients but following different recipes.
💡 Lab Tip: Benedict's test turning orange = reducing sugar present. Blue-black with iodine = starch present. These are essential tests to remember!

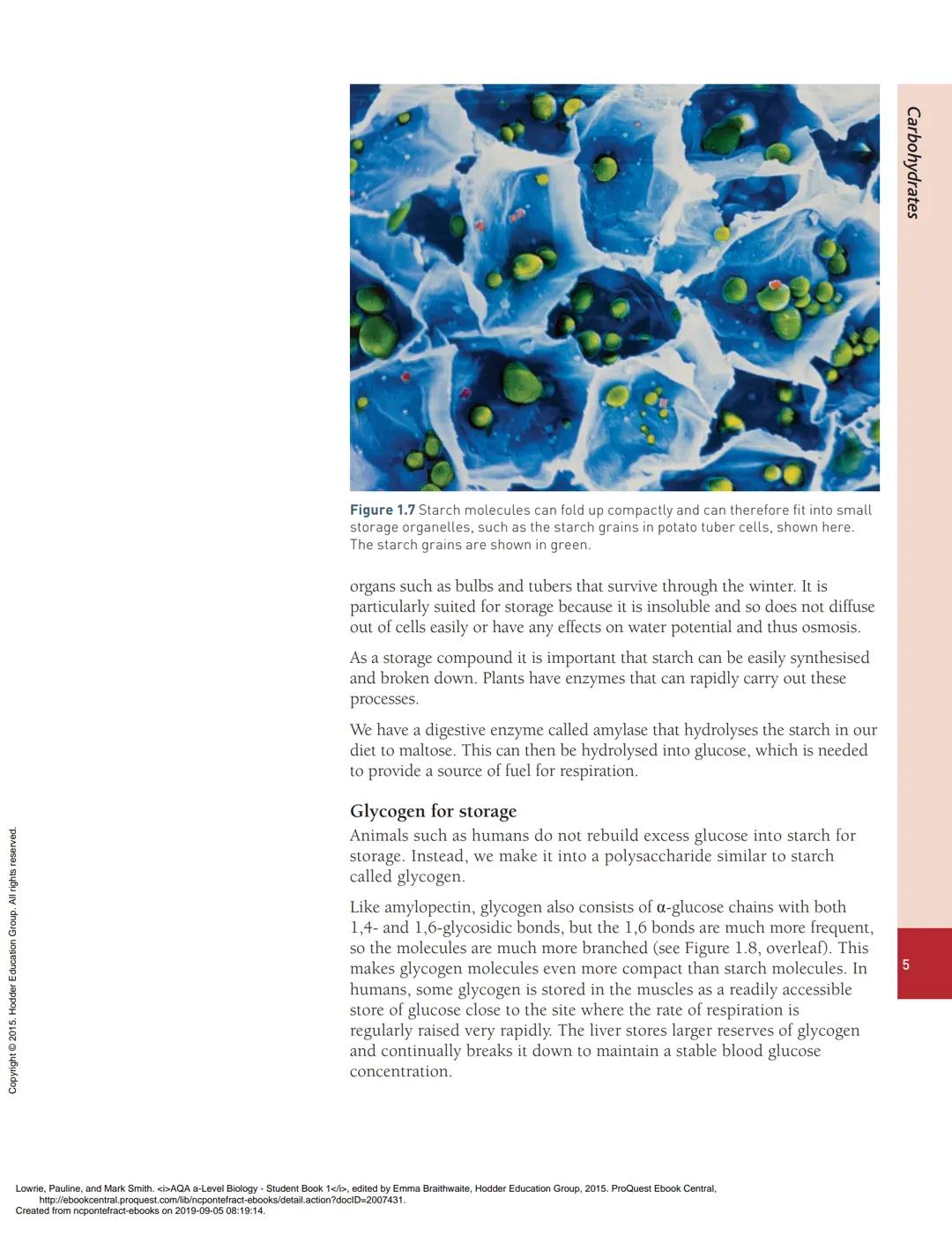
Starch is made from two components: amylose and amylopectin. Amylose forms long, straight chains of α-glucose that coil into spirals, held together by hydrogen bonds. Amylopectin has branches because some glucose molecules link differently (1,6 bonds instead of 1,4).
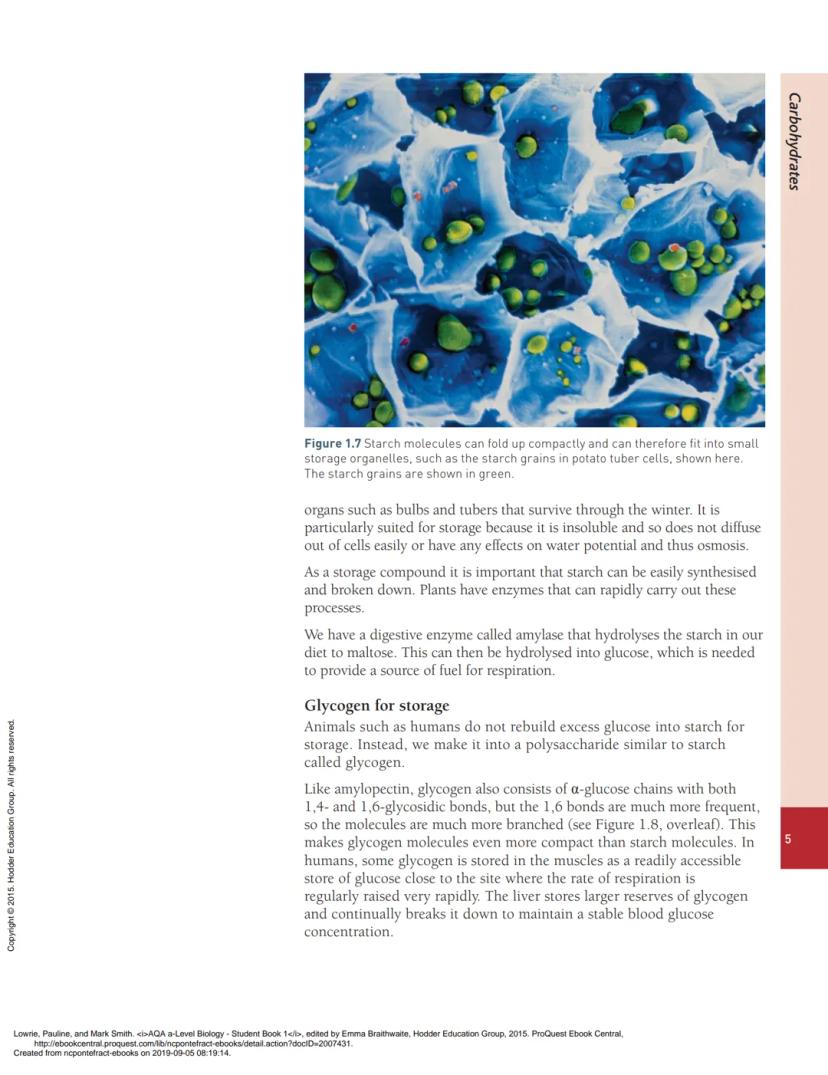
This branched structure makes starch incredibly compact - perfect for storage. Plants pack starch into tiny granules that don't take up much space but store loads of energy.
Starch is ideal for storage because it's insoluble (won't dissolve and leak out of cells) and doesn't affect osmosis. Plants can quickly build it up when they have excess glucose, and break it down when they need energy. Your digestive enzyme amylase breaks down dietary starch into maltose, which then gets converted to glucose for energy.
The coiled, compact structure means plants can store massive amounts of energy in small spaces - think of a potato or grain of rice packed full of starch granules.
💡 Storage Smart: Starch's insoluble nature means it won't mess with a cell's water balance whilst storing energy efficiently.
Glycogen is animals' version of starch - it's like amylopectin but with way more branches. This makes it even more compact and means it can be broken down super quickly when you need energy fast. Your muscles store glycogen locally for instant energy, whilst your liver maintains bigger reserves.
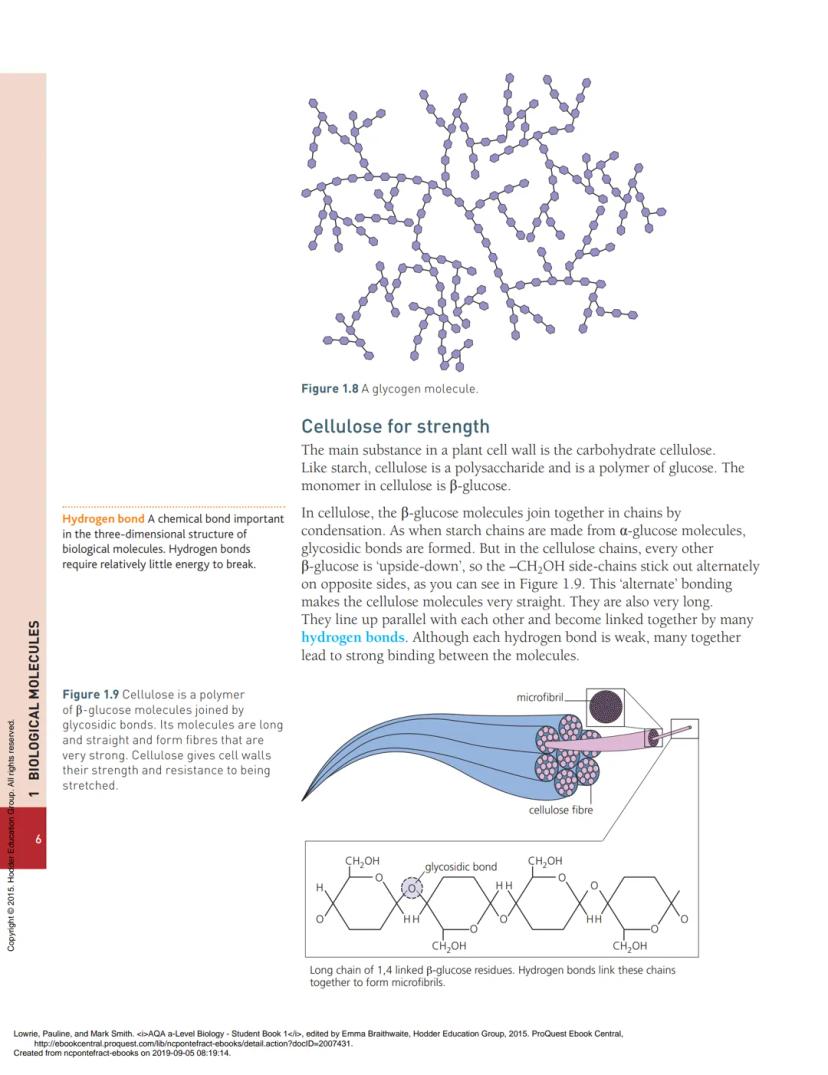
Cellulose is completely different even though it's also made from glucose. It uses β-glucose instead of α-glucose, and every other glucose molecule is flipped upside-down. This creates long, straight chains that line up parallel to each other.
Multiple hydrogen bonds between cellulose chains create microfibrils that are incredibly strong - as strong as steel fibres of the same thickness. These microfibrils criss-cross in plant cell walls, making them resistant to stretching in any direction.
Humans can't digest cellulose because we lack the right enzymes. Only certain bacteria and fungi can break it down, which is why cows need special gut bacteria to digest grass.
💡 Structure = Function: Glycogen's extra branches = faster energy release. Cellulose's straight, bonded chains = maximum strength.
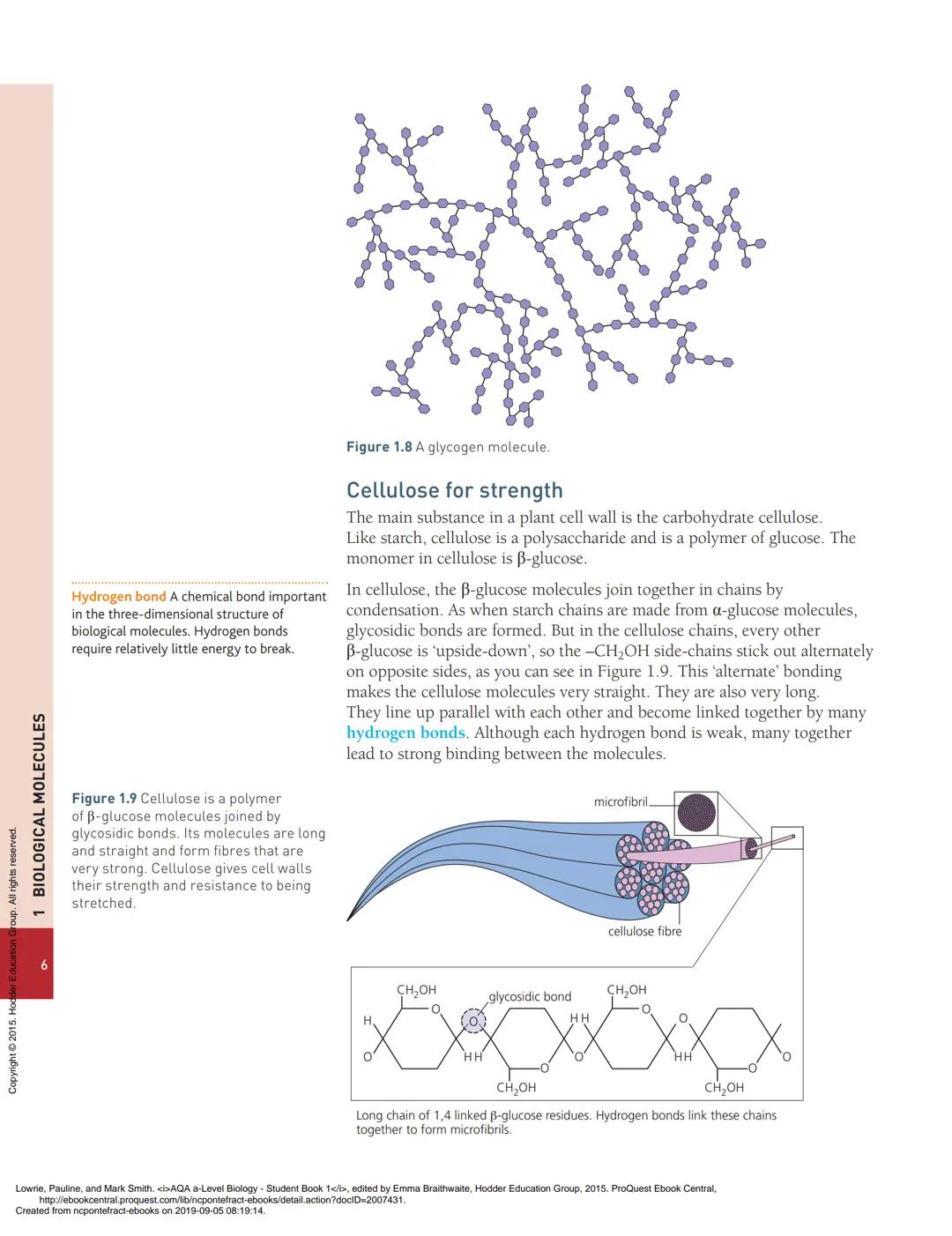
The alternating "upside-down" arrangement of β-glucose molecules in cellulose creates a unique structural advantage. Unlike the coiled chains of starch, cellulose forms perfectly straight chains with -CH₂OH groups sticking out on alternating sides.
These straight chains pack together tightly and are held by numerous hydrogen bonds. Although each individual hydrogen bond is weak, having thousands of them creates incredibly strong microfibrils. These bundle together to form even stronger fibres.
Plant cell walls use these fibres in a criss-cross pattern, creating walls that resist stretching in all directions. This gives plants their structural strength and allows them to grow tall and withstand environmental stresses like wind.
Cellulose is probably the most abundant carbohydrate on Earth. Without bacteria and fungi that can break it down, dead plant material would never decompose and the planet would be buried under cellulose waste.
💡 Amazing Fact: Cellulose microfibrils are as strong as steel wire of the same diameter - nature's incredible engineering!

Lipids include fats, oils, steroids, and waxes, but the most important ones you need to understand are triglycerides and phospholipids. Unlike carbohydrates and proteins, lipids aren't polymers - they're not made from repeating monomer units.
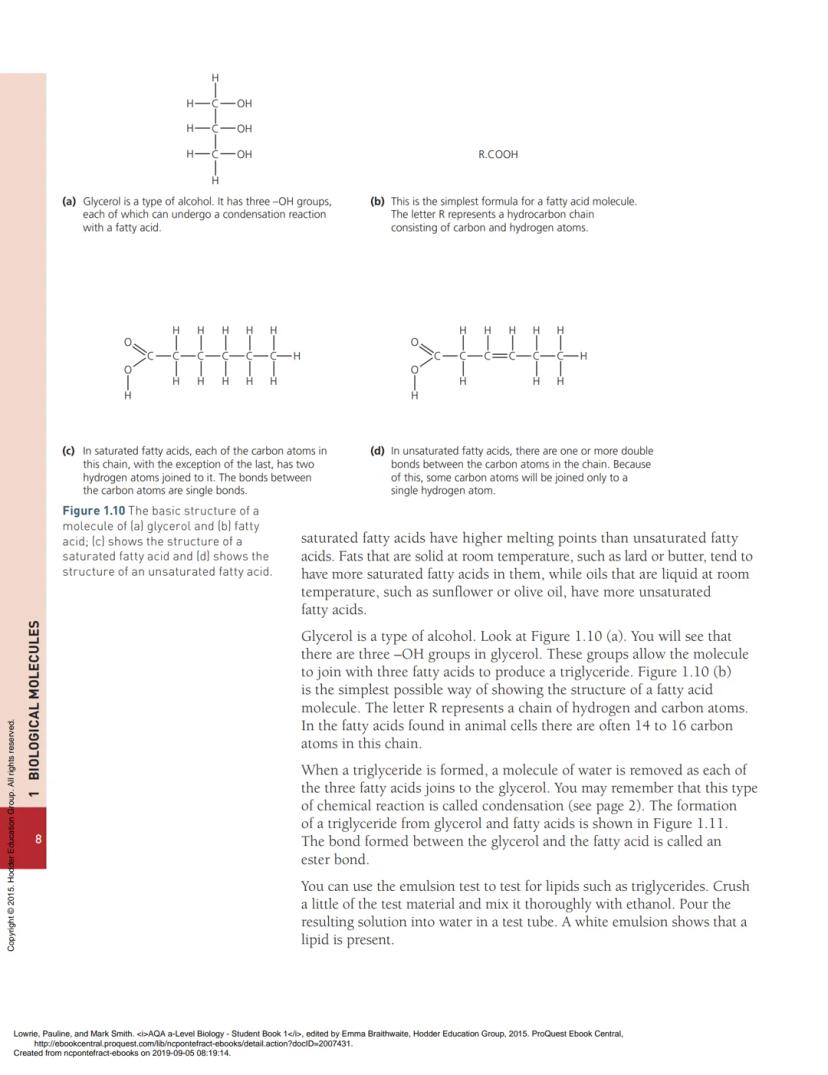
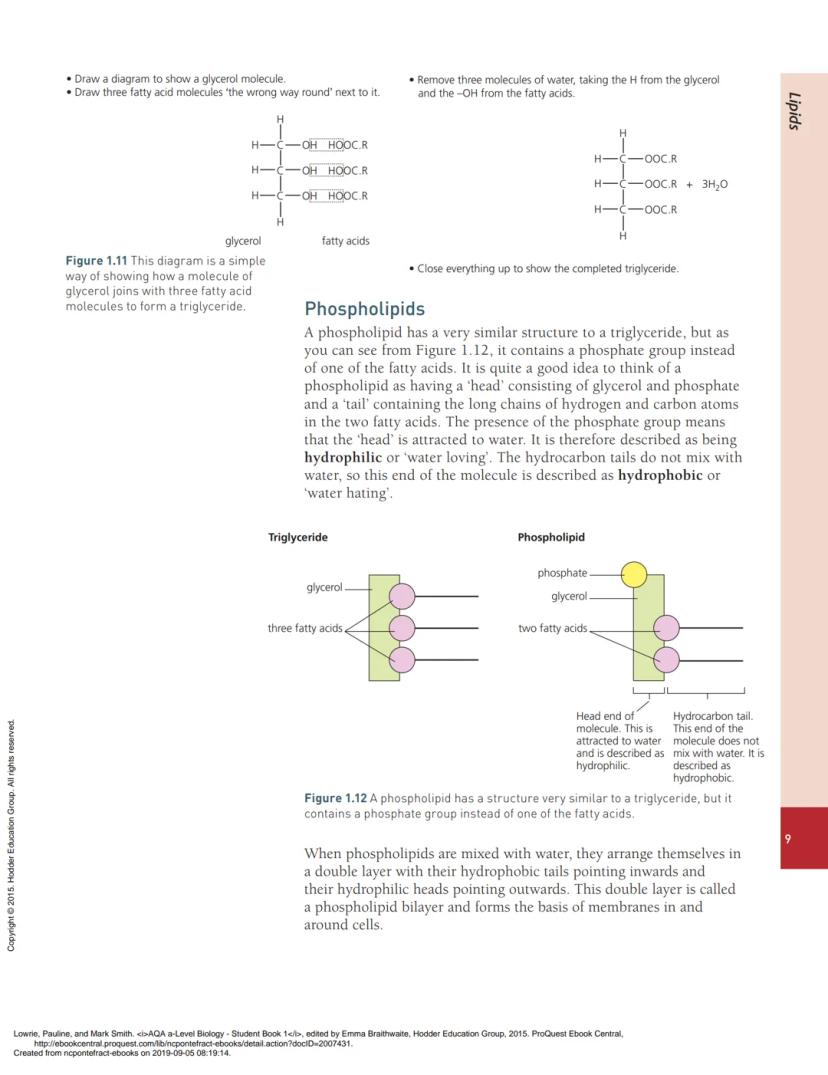
Triglycerides consist of one glycerol molecule joined to three fatty acid molecules. Glycerol has three -OH groups that can each bond with a fatty acid through condensation reactions, forming ester bonds.
Fatty acids come in two types: saturated (only single bonds between carbons) and unsaturated (one or more double bonds). Saturated fats are usually solid at room temperature (like butter), whilst unsaturated fats tend to be liquid (like olive oil).
The emulsion test identifies lipids - mix your sample with ethanol, then add water. If lipids are present, you'll see a white cloudy emulsion form.
💡 Memory Trick: Saturated fats are "saturated" with hydrogen atoms - they can't fit any more because all carbon bonds are single bonds.
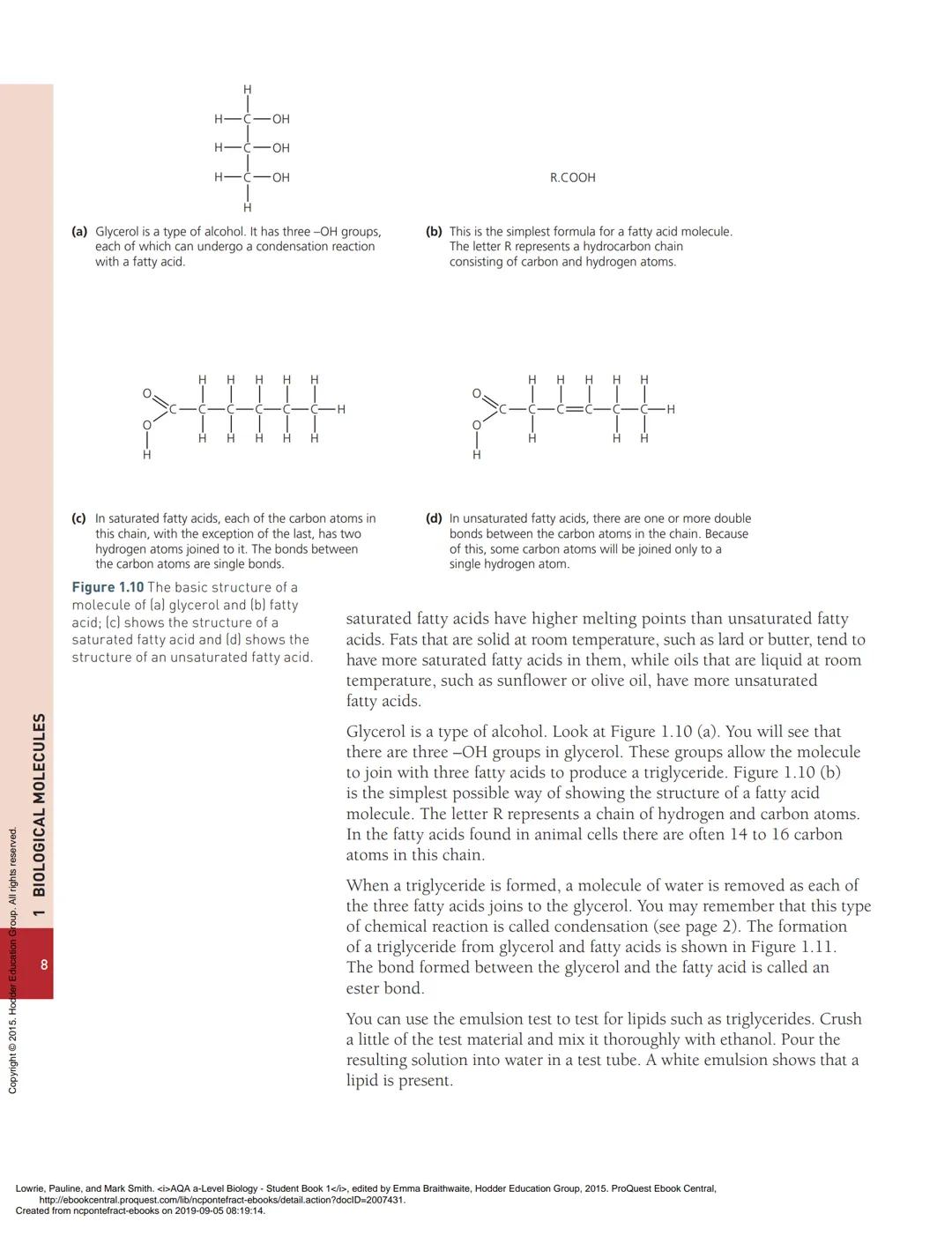
When triglycerides form, each of the three fatty acid molecules joins to glycerol through condensation. This removes three water molecules - one for each fatty acid that attaches. The bonds formed are called ester bonds.
The length of fatty acid chains in animal cells is typically 14-16 carbon atoms, but this can vary. Saturated fatty acids have higher melting points than unsaturated fatty acids because they can pack together more tightly.
Think of saturated fatty acids as straight chains that stack neatly, whilst unsaturated fatty acids have kinks from double bonds that prevent tight packing. This is why butter (more saturated) is solid whilst olive oil (more unsaturated) stays liquid at room temperature.
The structure directly affects function - solid fats provide structural support and insulation, whilst liquid oils are better for energy storage and cell membrane flexibility.
💡 Visual Learning: Draw glycerol with three -OH groups, then "flip" three fatty acids so their -COOH groups point toward the -OH groups. Remove water and connect!
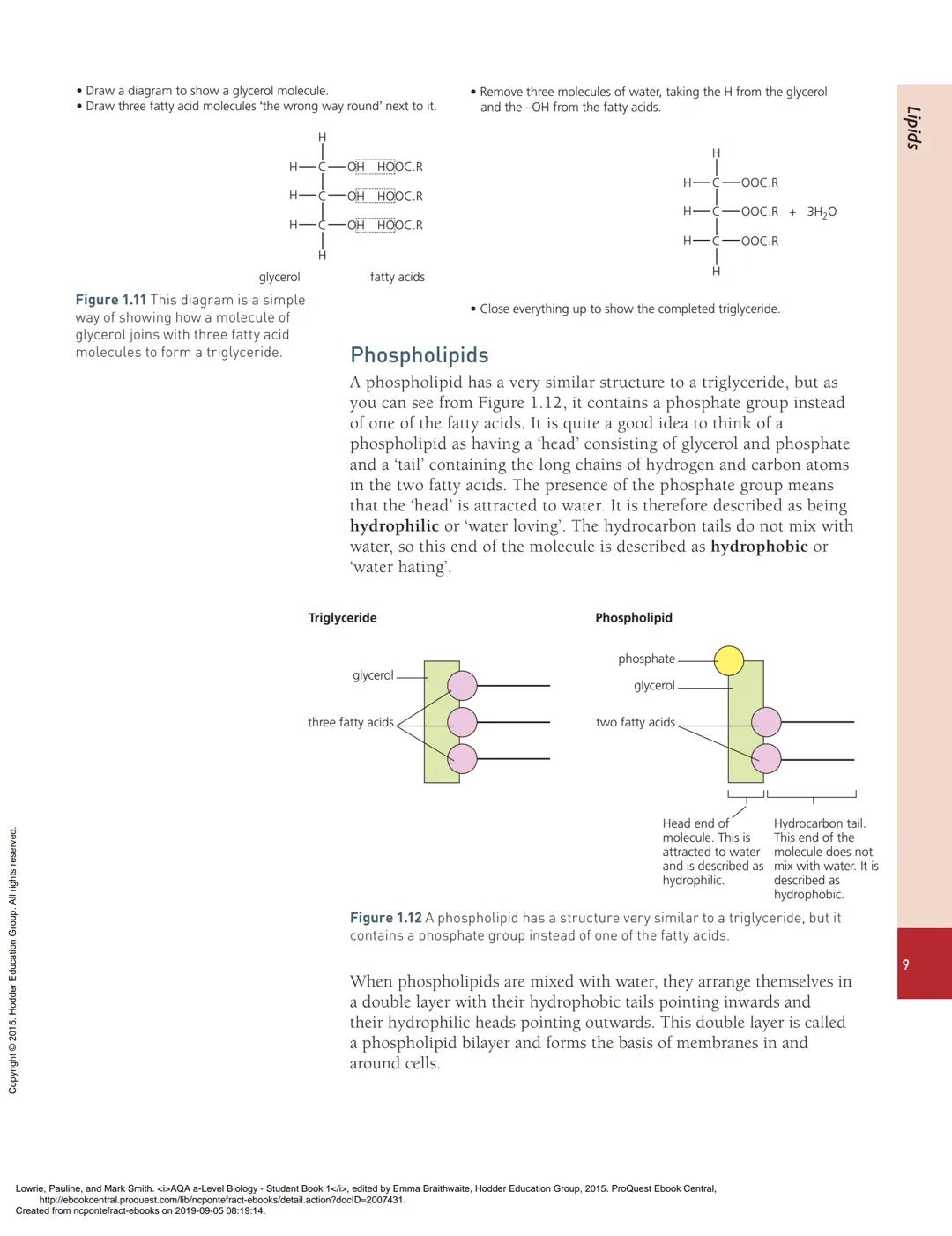
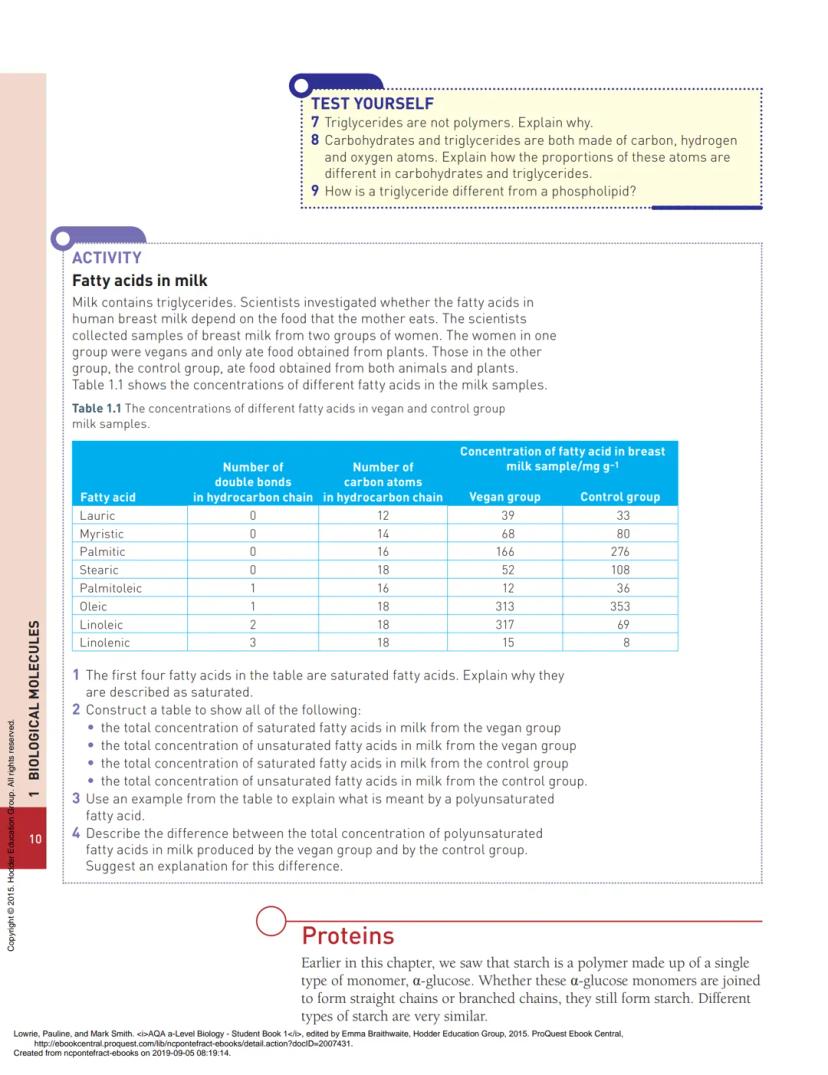
Phospholipids are similar to triglycerides but have a phosphate group instead of one fatty acid. This creates a molecule with two distinct ends: a hydrophilic "head" containing glycerol and phosphate, and hydrophobic "tails" made of fatty acid chains.
This dual nature is crucial for cell membranes. When phospholipids mix with water, they automatically arrange into a phospholipid bilayer - two layers with hydrophobic tails pointing inward and hydrophilic heads facing the watery environment on both sides.
This bilayer forms the foundation of all cell membranes, creating a barrier that separates the inside of cells from the outside environment. The hydrophobic interior prevents water-soluble substances from passing through easily, whilst the flexible structure allows the membrane to bend and reshape.
The phospholipid bilayer is like a molecular sandwich - water-loving bread on the outside, water-hating filling on the inside.
💡 Key Concept: Phospholipids self-assemble into membranes because of their amphipathic nature .
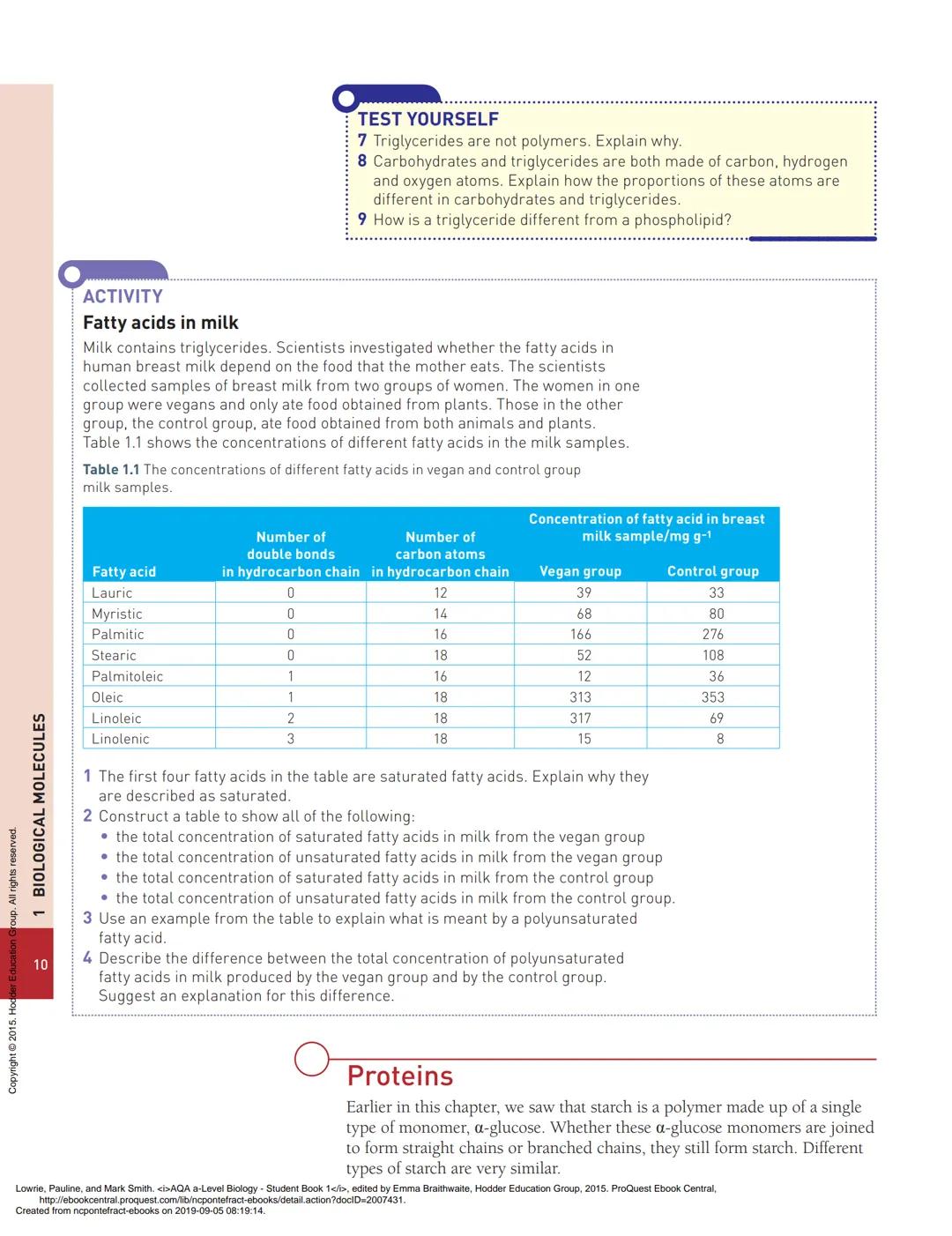
Understanding triglycerides helps explain nutrition and health. The table showing fatty acid concentrations in breast milk demonstrates how diet affects the lipids we produce. Polyunsaturated fatty acids (containing multiple double bonds) are essential nutrients that our bodies can't make.
Vegan mothers' milk contained more polyunsaturated fatty acids because plant-based diets are richer in these essential fats. This shows how molecular structure directly impacts nutrition and health outcomes.
Saturated fatty acids have no double bonds in their hydrocarbon chains - they're "saturated" with hydrogen atoms. Unsaturated fatty acids have one or more double bonds, and polyunsaturated means multiple double bonds.
The different properties of these fatty acids affect everything from food texture to cardiovascular health. Understanding their molecular structures helps you make sense of nutritional advice and food science.
💡 Real-World Connection: The fatty acid composition of foods directly relates to their physical properties and nutritional value - chemistry you can taste!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
Jasmin Thompson
@jasmintho_n26iu
Ever wondered what makes up all living things at the most basic level? From the tiniest bacteria to massive oak trees, all organisms share the same fundamental building blocks - biological molecules. These molecules might seem complex, but understanding them... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
All living organisms are remarkably similar when you look closely at their molecular structure. Whether you're examining a human, a houseplant, or bacteria, they're all built from the same types of biological molecules.
These molecules fall into two main categories: small molecules called monomers and massive molecules called polymers. Think of monomers as individual LEGO bricks, whilst polymers are the complete structures you build from joining loads of bricks together.
The most important groups you need to know are carbohydrates, lipids, and proteins. Each group has specific jobs - carbs provide energy, lipids store energy and form cell membranes, and proteins do almost everything else from building muscle to speeding up reactions.
💡 Key Point: Carbon atoms are special because they can form four bonds, allowing them to create the complex molecular frameworks that make life possible.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Macromolecules are built through a process called condensation, where two smaller molecules join together and kick out a water molecule in the process. It's like molecular matchmaking - one molecule loses a hydrogen (H), the other loses a hydroxyl group (OH), and together they form water (H₂O).
The opposite process is hydrolysis - breaking big molecules back down by adding water. Your digestive system uses hydrolysis constantly to break down food into smaller, usable pieces.
Carbohydrates contain carbon, hydrogen, and oxygen in a 2:1 ratio (twice as many hydrogens as oxygens). They come in three sizes: monosaccharides (single sugars like glucose), disaccharides (two sugars joined together like sucrose), and polysaccharides (massive chains like starch).
💡 Remember: Condensation reactions build molecules UP by removing water, whilst hydrolysis breaks molecules DOWN by adding water.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Glucose has the formula C₆H₁₂O₆, but there are actually two different versions: α-glucose and β-glucose. These are called isomers - same formula, different arrangement of atoms. The difference is tiny but crucial for how they behave.
When two glucose molecules join, they form a glycosidic bond through condensation. Two α-glucose molecules create maltose, whilst glucose can also join with other sugars like fructose to make sucrose (table sugar).
Reducing sugars like glucose, fructose, and maltose will turn Benedict's solution orange when heated - this happens because they can donate electrons (get reduced). Sucrose won't do this directly, but if you break it down with acid first, then it will test positive.
Different sugars have identical formulas but completely different properties because of how their atoms are arranged. It's like having the same ingredients but following different recipes.
💡 Lab Tip: Benedict's test turning orange = reducing sugar present. Blue-black with iodine = starch present. These are essential tests to remember!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Starch is made from two components: amylose and amylopectin. Amylose forms long, straight chains of α-glucose that coil into spirals, held together by hydrogen bonds. Amylopectin has branches because some glucose molecules link differently (1,6 bonds instead of 1,4).
This branched structure makes starch incredibly compact - perfect for storage. Plants pack starch into tiny granules that don't take up much space but store loads of energy.
Starch is ideal for storage because it's insoluble (won't dissolve and leak out of cells) and doesn't affect osmosis. Plants can quickly build it up when they have excess glucose, and break it down when they need energy. Your digestive enzyme amylase breaks down dietary starch into maltose, which then gets converted to glucose for energy.
The coiled, compact structure means plants can store massive amounts of energy in small spaces - think of a potato or grain of rice packed full of starch granules.
💡 Storage Smart: Starch's insoluble nature means it won't mess with a cell's water balance whilst storing energy efficiently.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Glycogen is animals' version of starch - it's like amylopectin but with way more branches. This makes it even more compact and means it can be broken down super quickly when you need energy fast. Your muscles store glycogen locally for instant energy, whilst your liver maintains bigger reserves.
Cellulose is completely different even though it's also made from glucose. It uses β-glucose instead of α-glucose, and every other glucose molecule is flipped upside-down. This creates long, straight chains that line up parallel to each other.
Multiple hydrogen bonds between cellulose chains create microfibrils that are incredibly strong - as strong as steel fibres of the same thickness. These microfibrils criss-cross in plant cell walls, making them resistant to stretching in any direction.
Humans can't digest cellulose because we lack the right enzymes. Only certain bacteria and fungi can break it down, which is why cows need special gut bacteria to digest grass.
💡 Structure = Function: Glycogen's extra branches = faster energy release. Cellulose's straight, bonded chains = maximum strength.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The alternating "upside-down" arrangement of β-glucose molecules in cellulose creates a unique structural advantage. Unlike the coiled chains of starch, cellulose forms perfectly straight chains with -CH₂OH groups sticking out on alternating sides.
These straight chains pack together tightly and are held by numerous hydrogen bonds. Although each individual hydrogen bond is weak, having thousands of them creates incredibly strong microfibrils. These bundle together to form even stronger fibres.
Plant cell walls use these fibres in a criss-cross pattern, creating walls that resist stretching in all directions. This gives plants their structural strength and allows them to grow tall and withstand environmental stresses like wind.
Cellulose is probably the most abundant carbohydrate on Earth. Without bacteria and fungi that can break it down, dead plant material would never decompose and the planet would be buried under cellulose waste.
💡 Amazing Fact: Cellulose microfibrils are as strong as steel wire of the same diameter - nature's incredible engineering!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Lipids include fats, oils, steroids, and waxes, but the most important ones you need to understand are triglycerides and phospholipids. Unlike carbohydrates and proteins, lipids aren't polymers - they're not made from repeating monomer units.
Triglycerides consist of one glycerol molecule joined to three fatty acid molecules. Glycerol has three -OH groups that can each bond with a fatty acid through condensation reactions, forming ester bonds.
Fatty acids come in two types: saturated (only single bonds between carbons) and unsaturated (one or more double bonds). Saturated fats are usually solid at room temperature (like butter), whilst unsaturated fats tend to be liquid (like olive oil).
The emulsion test identifies lipids - mix your sample with ethanol, then add water. If lipids are present, you'll see a white cloudy emulsion form.
💡 Memory Trick: Saturated fats are "saturated" with hydrogen atoms - they can't fit any more because all carbon bonds are single bonds.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When triglycerides form, each of the three fatty acid molecules joins to glycerol through condensation. This removes three water molecules - one for each fatty acid that attaches. The bonds formed are called ester bonds.
The length of fatty acid chains in animal cells is typically 14-16 carbon atoms, but this can vary. Saturated fatty acids have higher melting points than unsaturated fatty acids because they can pack together more tightly.
Think of saturated fatty acids as straight chains that stack neatly, whilst unsaturated fatty acids have kinks from double bonds that prevent tight packing. This is why butter (more saturated) is solid whilst olive oil (more unsaturated) stays liquid at room temperature.
The structure directly affects function - solid fats provide structural support and insulation, whilst liquid oils are better for energy storage and cell membrane flexibility.
💡 Visual Learning: Draw glycerol with three -OH groups, then "flip" three fatty acids so their -COOH groups point toward the -OH groups. Remove water and connect!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Phospholipids are similar to triglycerides but have a phosphate group instead of one fatty acid. This creates a molecule with two distinct ends: a hydrophilic "head" containing glycerol and phosphate, and hydrophobic "tails" made of fatty acid chains.
This dual nature is crucial for cell membranes. When phospholipids mix with water, they automatically arrange into a phospholipid bilayer - two layers with hydrophobic tails pointing inward and hydrophilic heads facing the watery environment on both sides.
This bilayer forms the foundation of all cell membranes, creating a barrier that separates the inside of cells from the outside environment. The hydrophobic interior prevents water-soluble substances from passing through easily, whilst the flexible structure allows the membrane to bend and reshape.
The phospholipid bilayer is like a molecular sandwich - water-loving bread on the outside, water-hating filling on the inside.
💡 Key Concept: Phospholipids self-assemble into membranes because of their amphipathic nature .

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Understanding triglycerides helps explain nutrition and health. The table showing fatty acid concentrations in breast milk demonstrates how diet affects the lipids we produce. Polyunsaturated fatty acids (containing multiple double bonds) are essential nutrients that our bodies can't make.
Vegan mothers' milk contained more polyunsaturated fatty acids because plant-based diets are richer in these essential fats. This shows how molecular structure directly impacts nutrition and health outcomes.
Saturated fatty acids have no double bonds in their hydrocarbon chains - they're "saturated" with hydrogen atoms. Unsaturated fatty acids have one or more double bonds, and polyunsaturated means multiple double bonds.
The different properties of these fatty acids affect everything from food texture to cardiovascular health. Understanding their molecular structures helps you make sense of nutritional advice and food science.
💡 Real-World Connection: The fatty acid composition of foods directly relates to their physical properties and nutritional value - chemistry you can taste!
Our AI Companion is a student-focused AI tool that offers more than just answers. Built on millions of Knowunity resources, it provides relevant information, personalised study plans, quizzes, and content directly in the chat, adapting to your individual learning journey.
You can download the app from Google Play Store and Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
0
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
Best app on earth! no words because it’s too good
Thomas R
iOS user
Just amazing. Let's me revise 10x better, this app is a quick 10/10. I highly recommend it to anyone. I can watch and search for notes. I can save them in the subject folder. I can revise it any time when I come back. If you haven't tried this app, you're really missing out.
Basil
Android user
This app has made me feel so much more confident in my exam prep, not only through boosting my own self confidence through the features that allow you to connect with others and feel less alone, but also through the way the app itself is centred around making you feel better. It is easy to navigate, fun to use, and helpful to anyone struggling in absolutely any way.
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
very reliable app to help and grow your ideas of Maths, English and other related topics in your works. please use this app if your struggling in areas, this app is key for that. wish I'd of done a review before. and it's also free so don't worry about that.
Rohan U
Android user
I know a lot of apps use fake accounts to boost their reviews but this app deserves it all. Originally I was getting 4 in my English exams and this time I got a grade 7. I didn’t even know about this app three days until the exam and it has helped A LOT. Please actually trust me and use it as I’m sure you too will see developments.
Xander S
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This apps acc the goat. I find revision so boring but this app makes it so easy to organize it all and then you can ask the freeeee ai to test yourself so good and you can easily upload your own stuff. highly recommend as someone taking mocks now
Paul T
iOS user